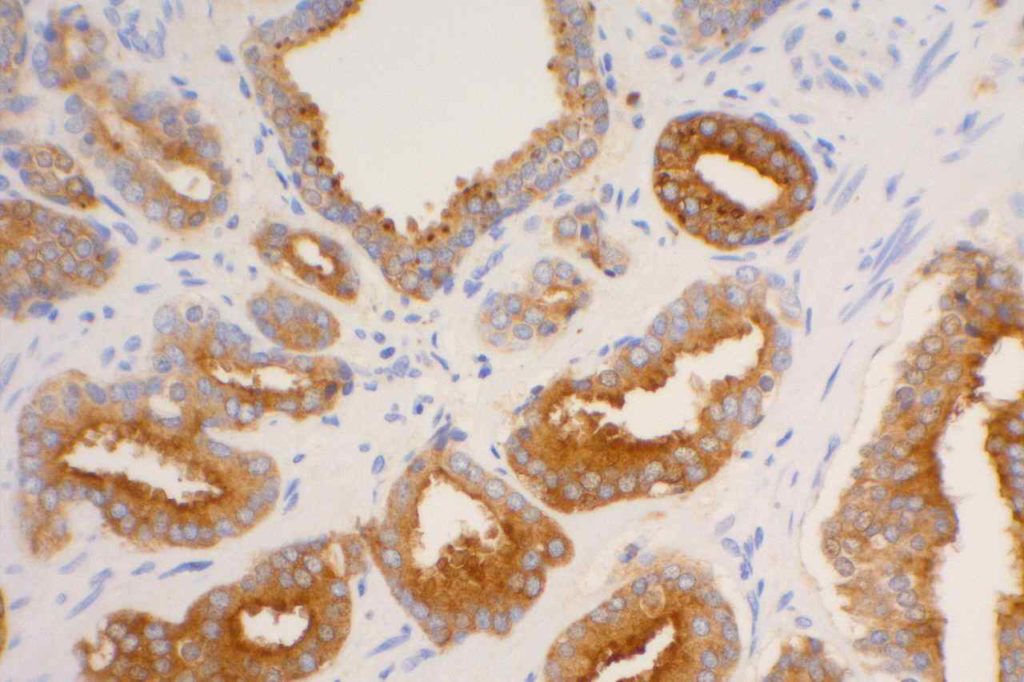
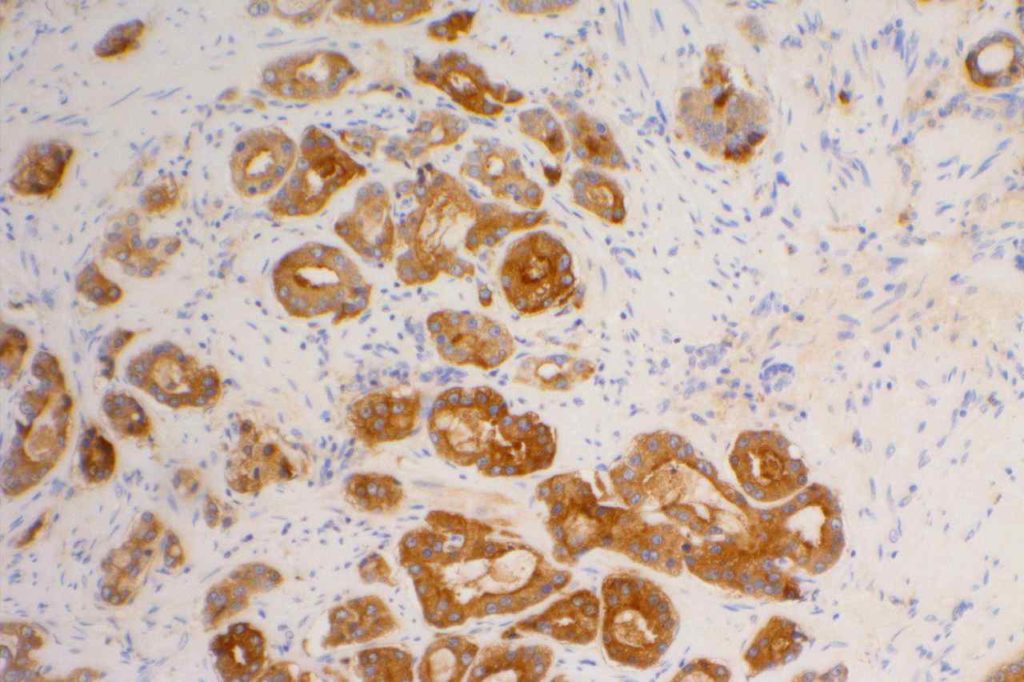
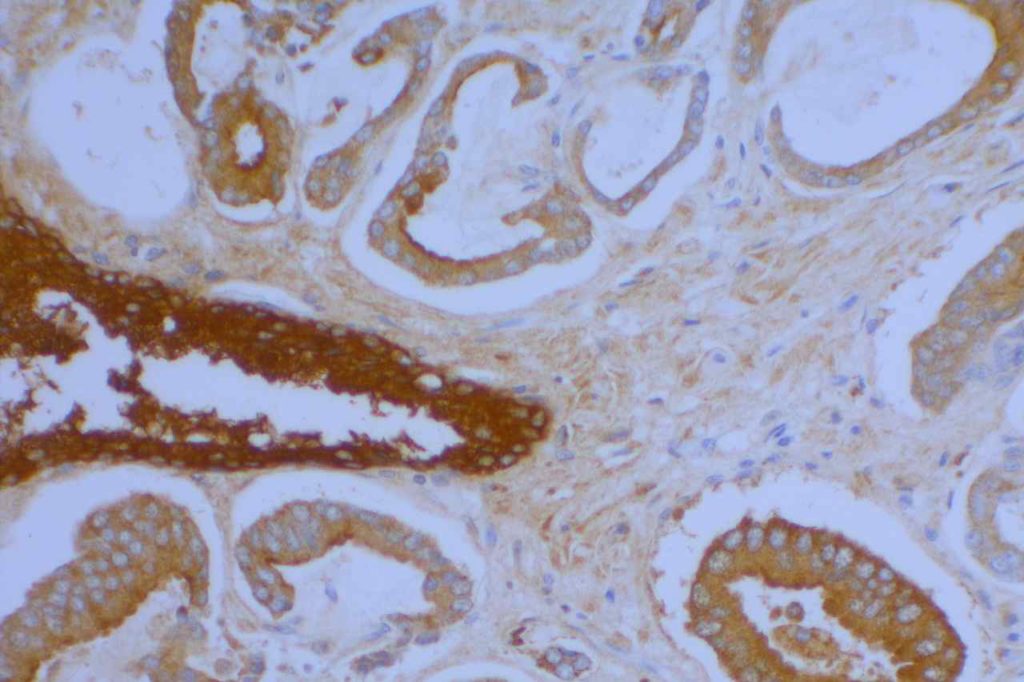
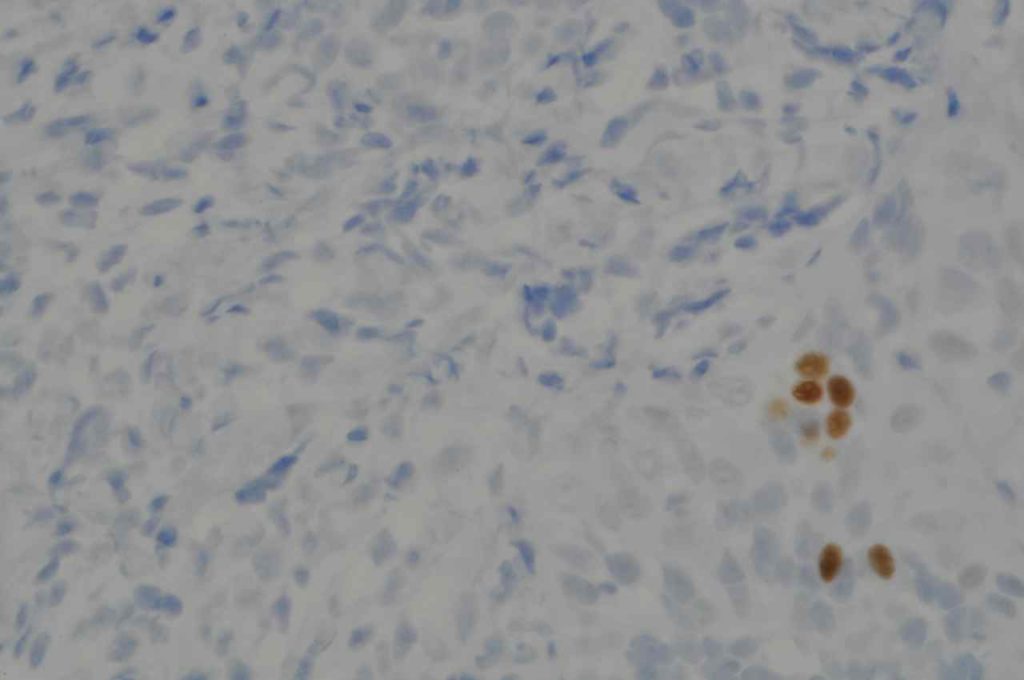
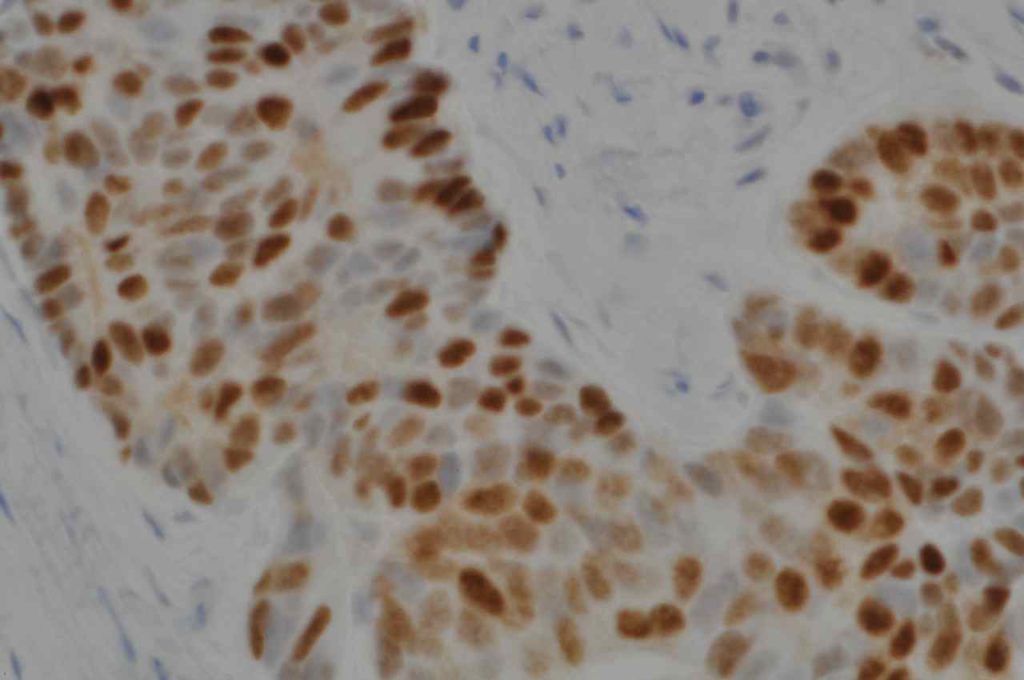
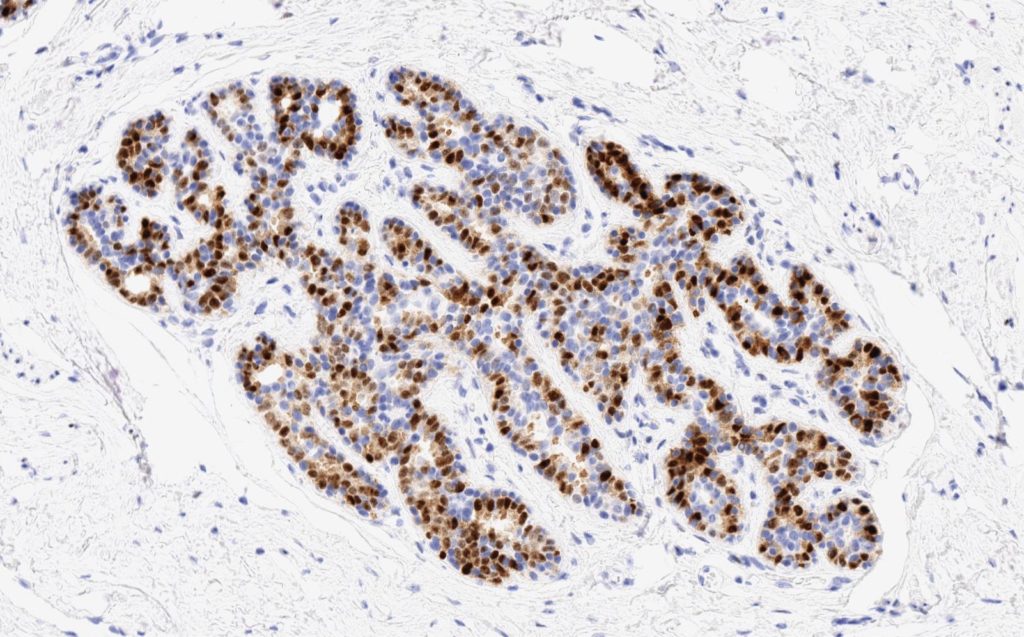
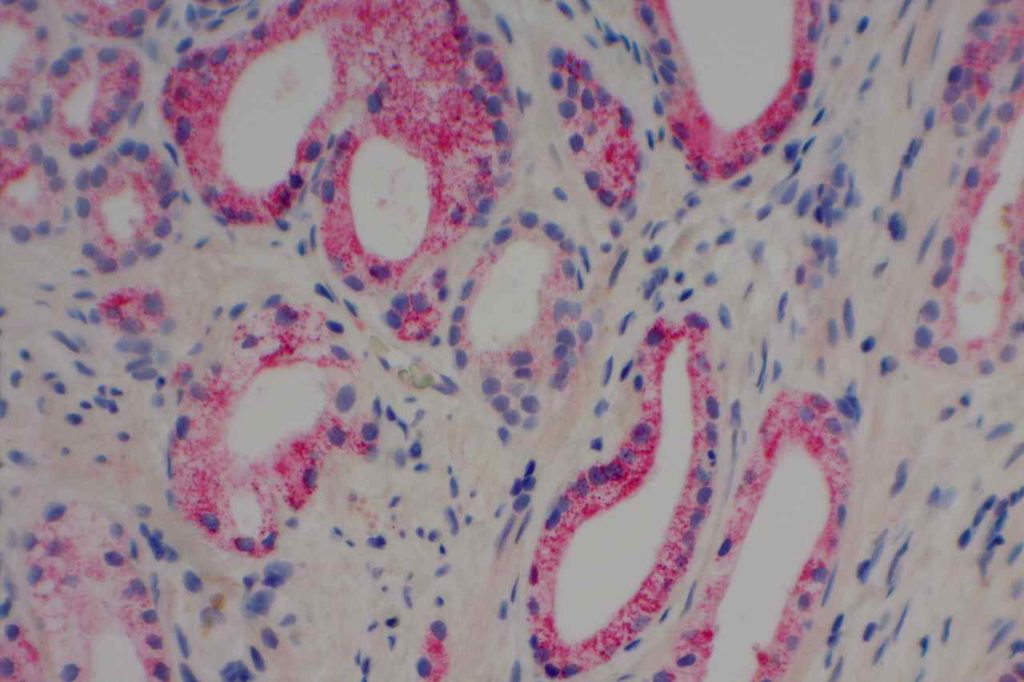
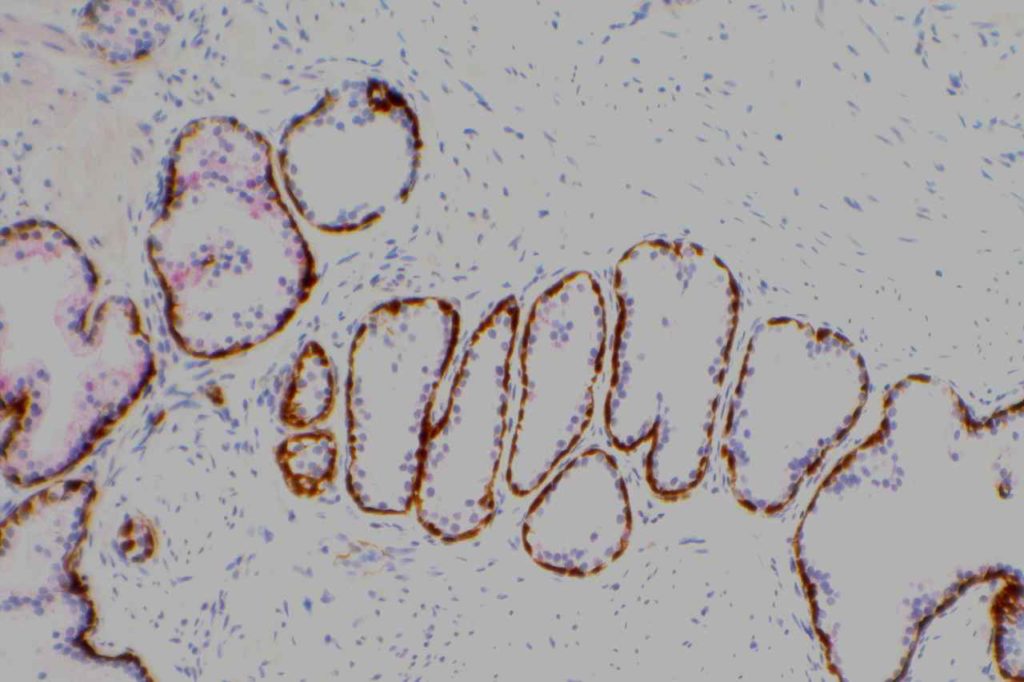
S100P (placental S100) is a member of the S100 family. It has been identified in human placenta and is expressed in a multitude of cancer types including: bladder, breast, esophagus, colon, lung, pancreatic, ovarian, hepatocellular (HCC), cholangiocarcinoma, and prostate (Yuan et al.). Interesting, Mohanty, et al. found S100P to be a relatively sensitive and specific marker for urothelial carcinoma in the differential of urothelial carcinoma vs. poorly differentiated prostate adenocarcinoma of the bladder neck (88% sensitive, 100% specific, N=16).
Practically, S100P may have some useful benefit in a limited differential setting, but given its general lack of specificity would probably not be significantly helpful in the setting of a large differential diagnosis.
Yuan, et al. did find S100P expression to be an independent poor prognostic indicator in HCC with early tumor recurrence or high tumor stage.
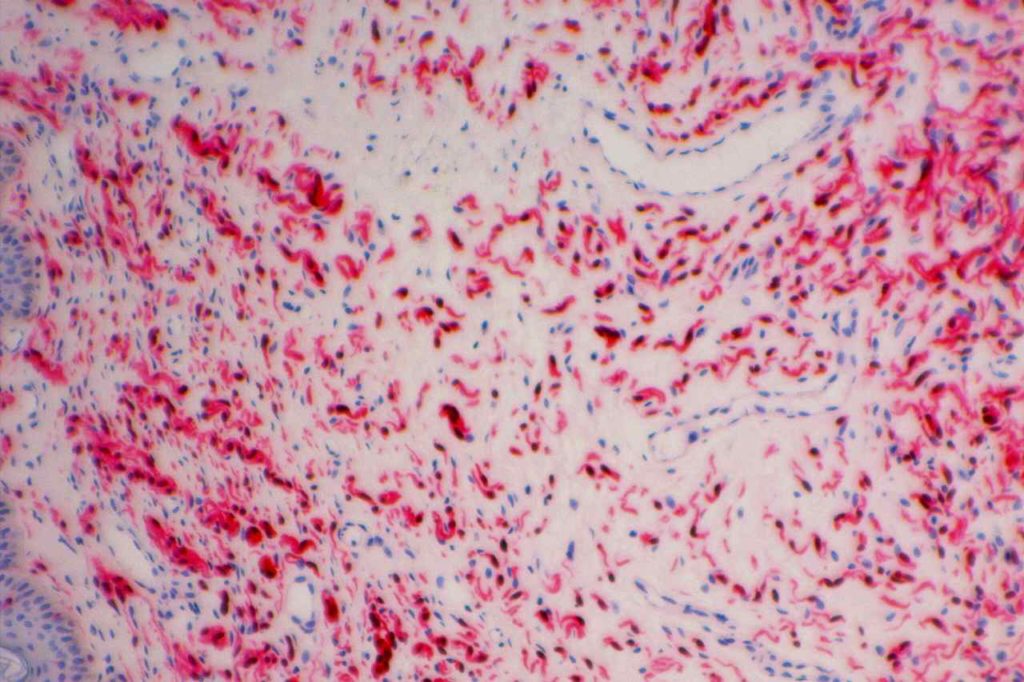
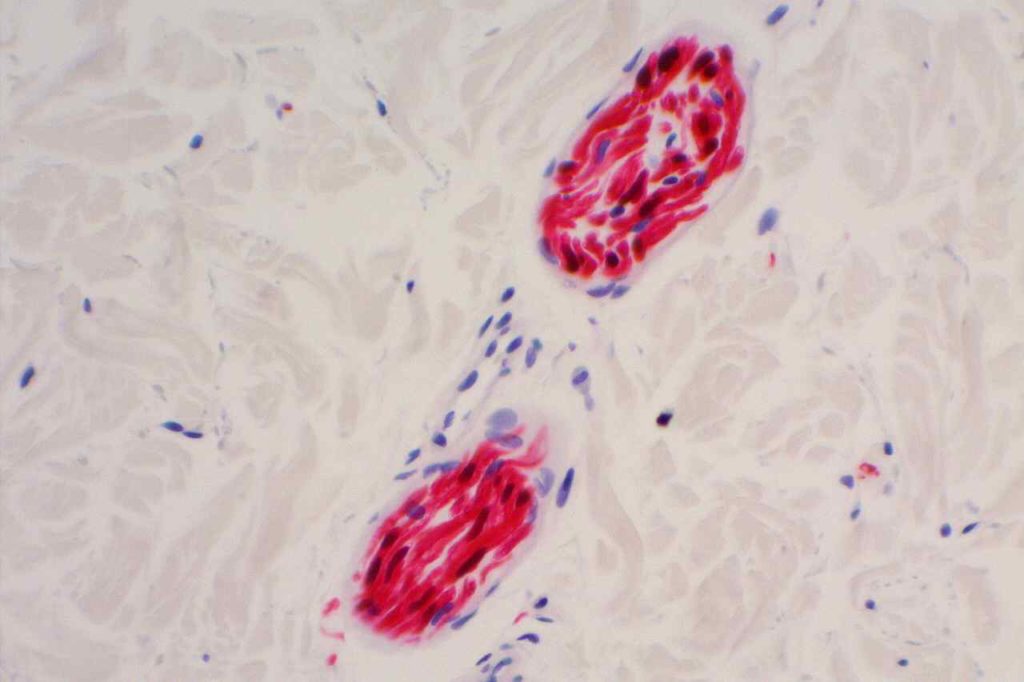
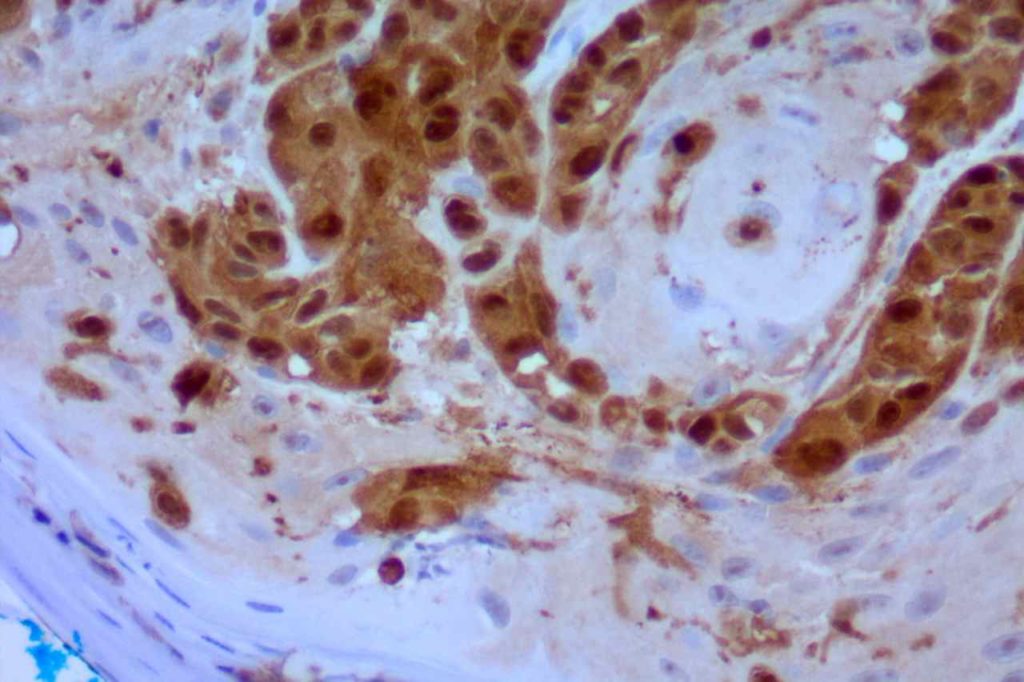
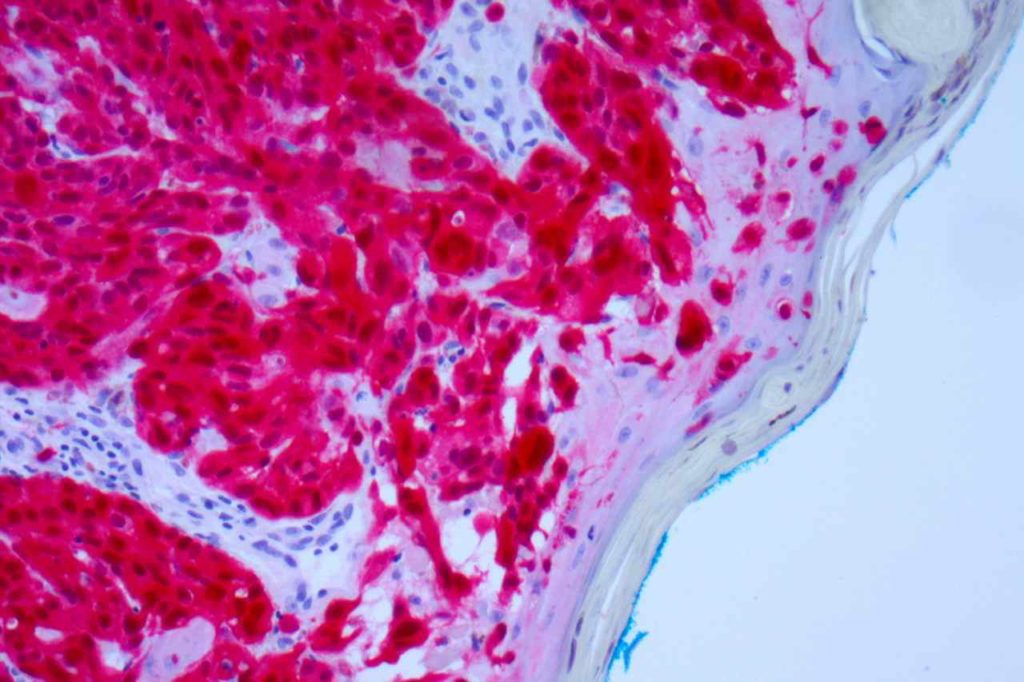
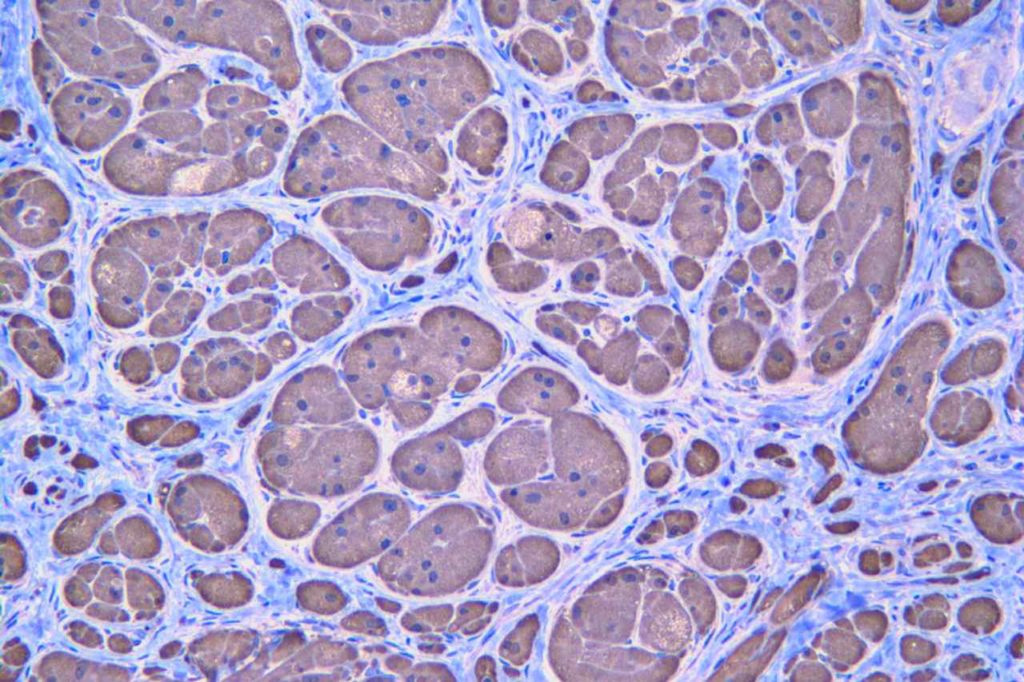
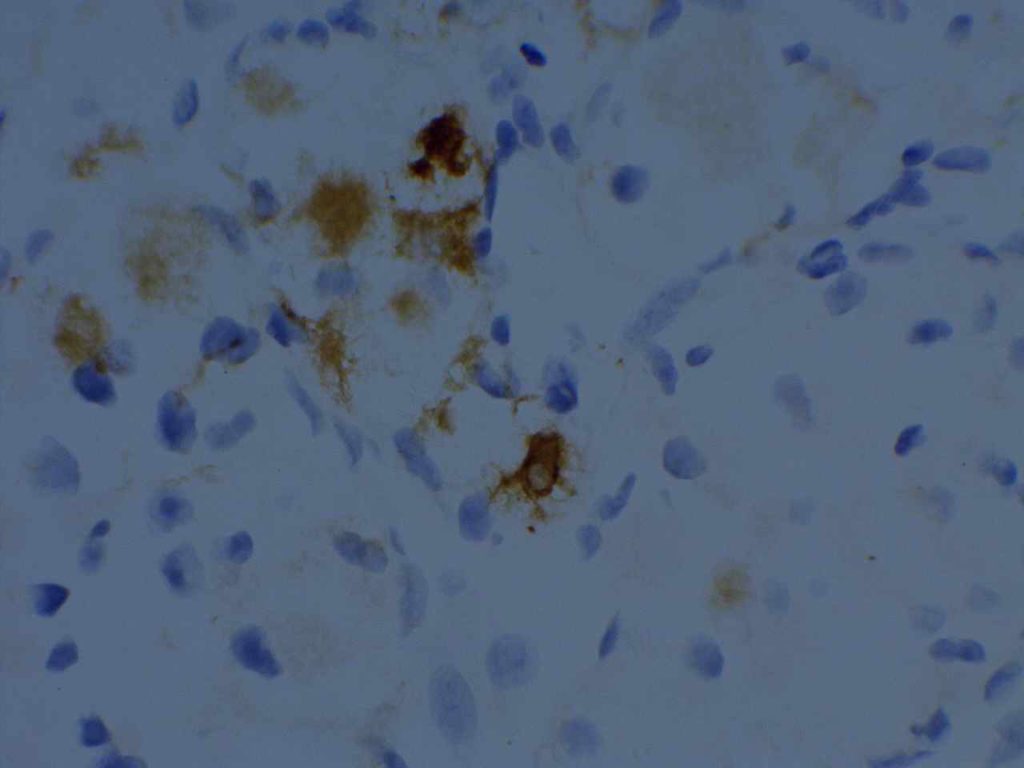
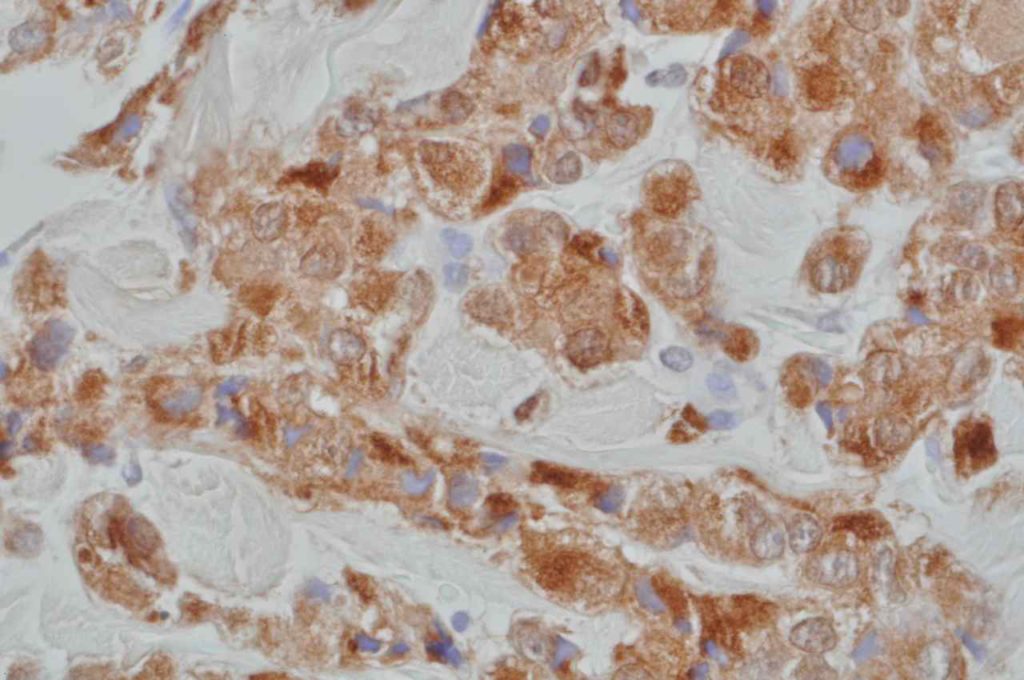
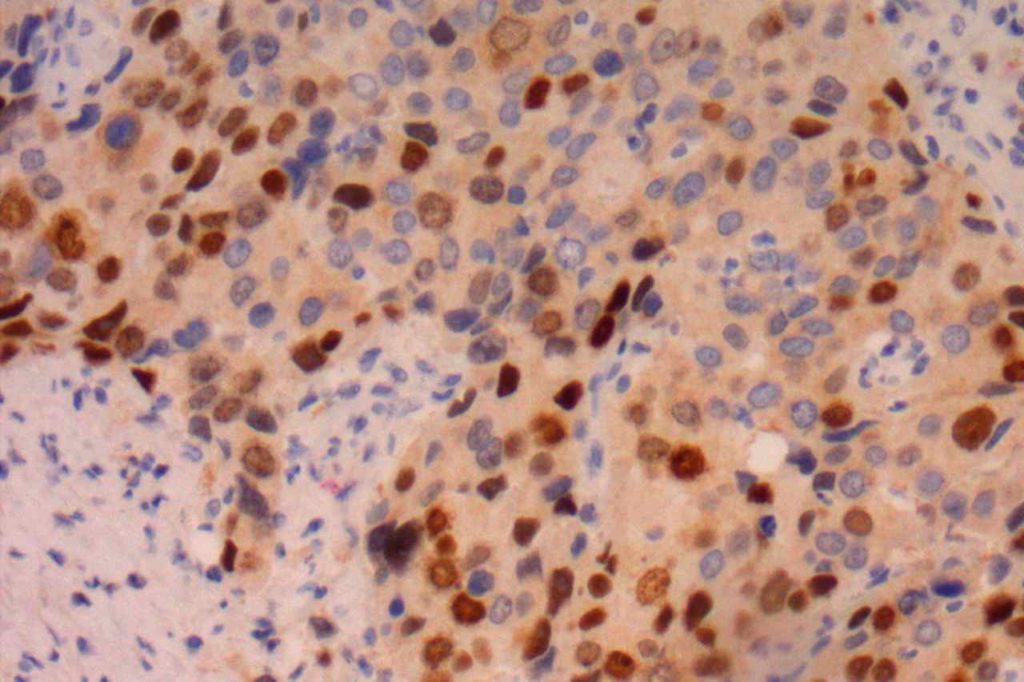
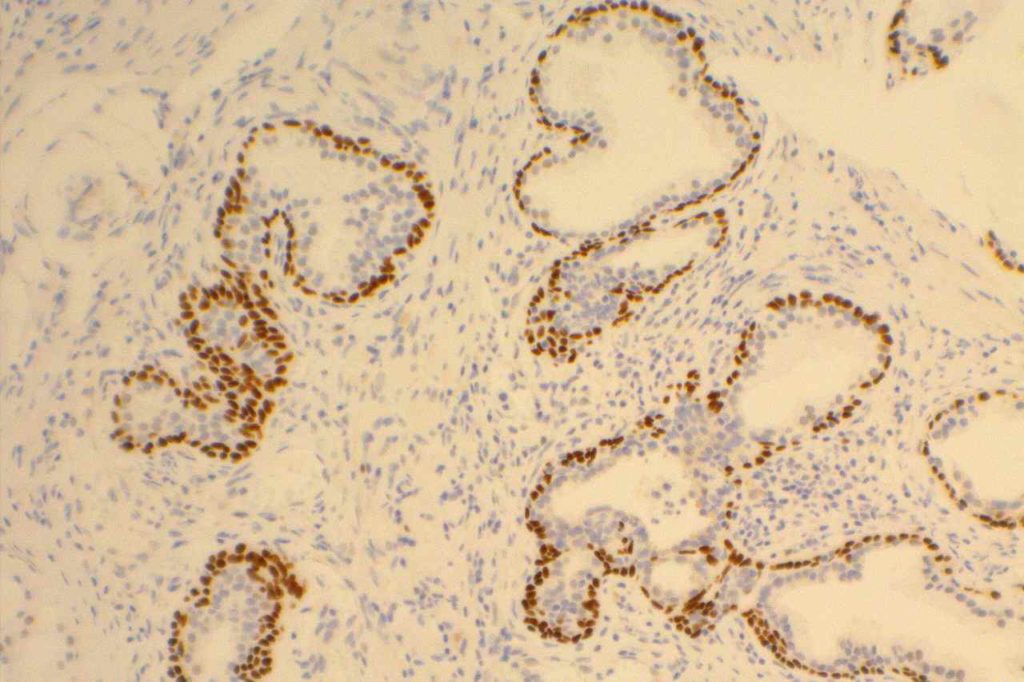
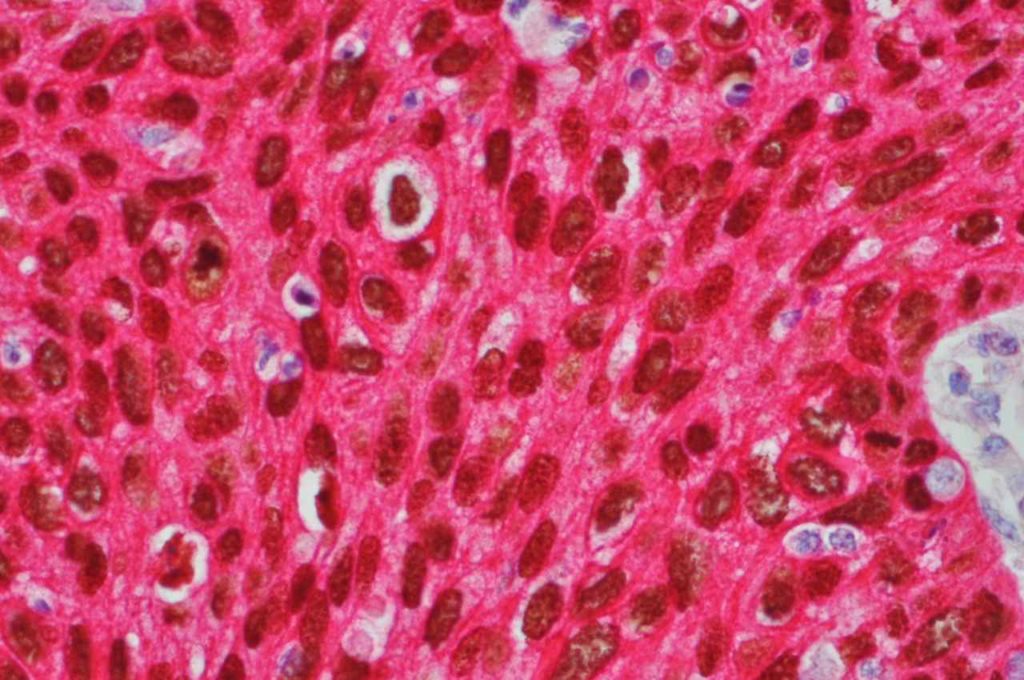
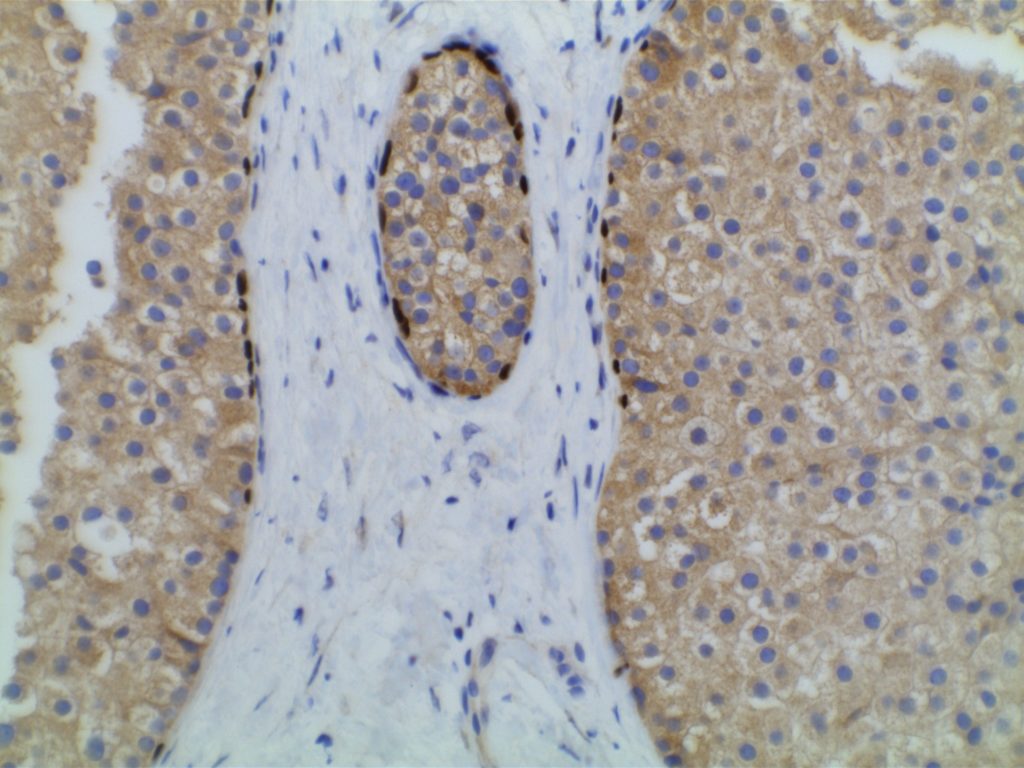
Microscopic Images
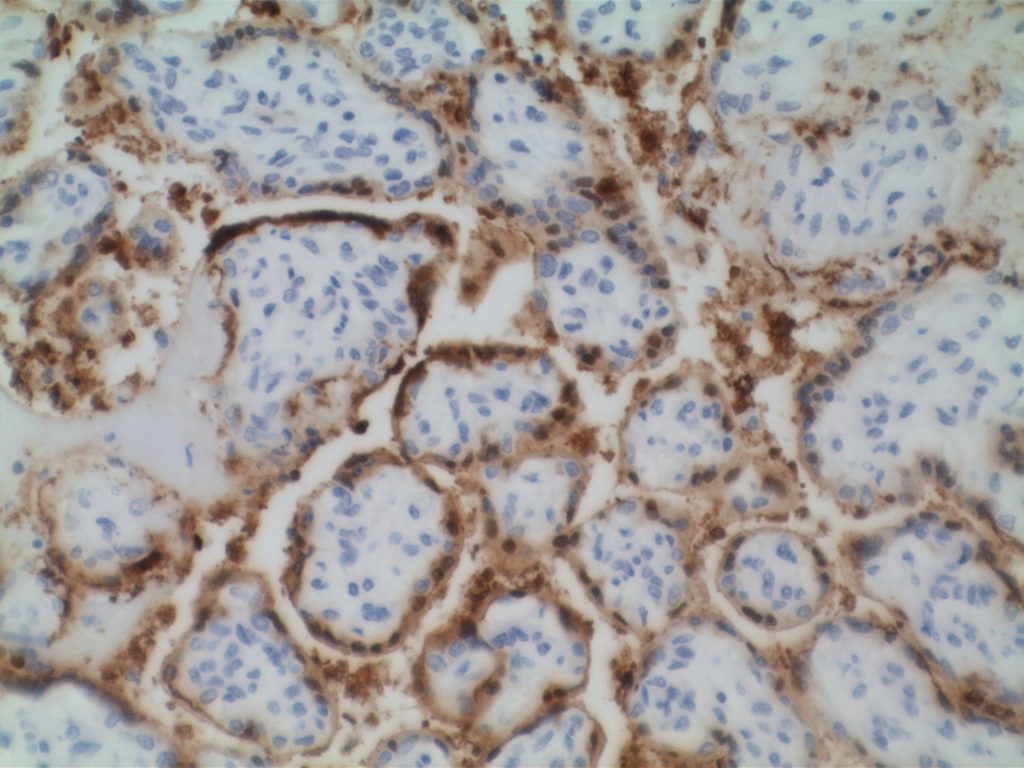
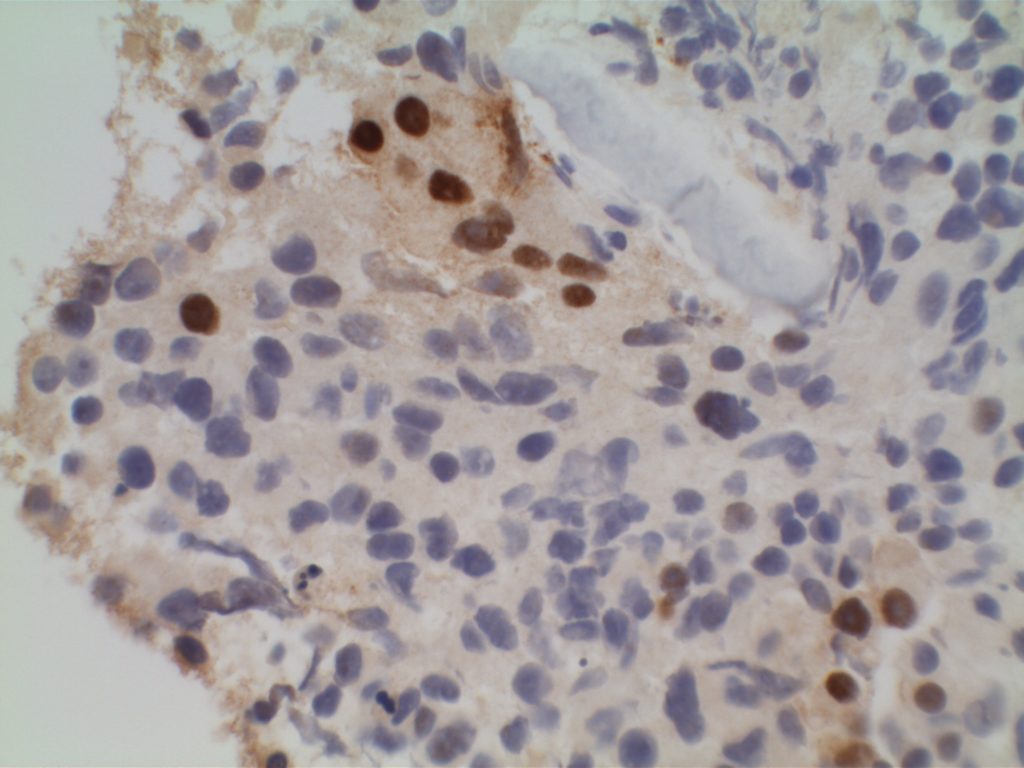
References
Yuan, R.-H., Chang, K.-T., Chen, Y.-L., Hsu, H.-C., Lee, P.-H., Lai, P.-L., & Jeng, Y.-M. (2013). S100P expression is a novel prognostic factor in hepatocellular carcinoma and predicts survival in patients with high tumor stage or early recurrent tumors. PloS One, 8(6), e65501. doi:10.1371/journal.pone.0065501
Mohanty SK, Smith SC, Chang E, et al. Evaluation of contemporary prostate and urothelial lineage biomarkers in a consecutive cohort of poorly differentiated bladder neck carcinomas. Am J Clin Pathol. 2014;142(2):173–183. doi:10.1309/AJCPK1OV6IMNPFGL.