Calcitonin is a neuroendocrine marker expressed in medullary carcinoma of the thyroid, and thus makes up the great majority of use in diagnostic immunohistochemistry. Continue reading Calcitonin
Category Archives: A – F Antibodies
Estrogen Receptor (ER)
Summary (Breast Carcinoma)
- Nuclear Marker
- Stain is reported as PERCENT STAINING OF TUMOR CELLS and STAIN INTENSITY (1+, 2+, 3+)
- 1% or greater nuclear expression in tumor cells is considered positive, and therefore eligible to receive hormonal therapy.
- CAP-ASCO recommendations are for <1 hr. from time of excision/biopsy to having a cut edge of tumor in 10% neutral bufferedormalin fixative. Fixation window of 6-72 hrs. These times should be noted in the pathology report (time of excision, time in gross room, and time in fixative).
- Negative staining results in biopsy material without an internal control should be repeated on the excisional specimen using blocks with both tumor and benign breast parenchyma.
General
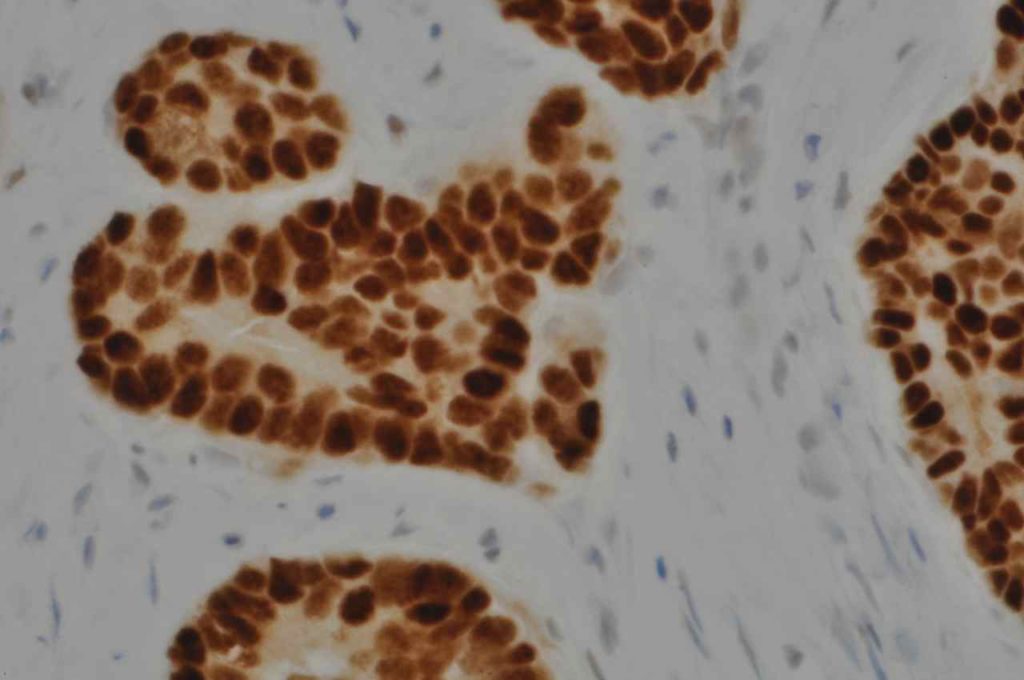
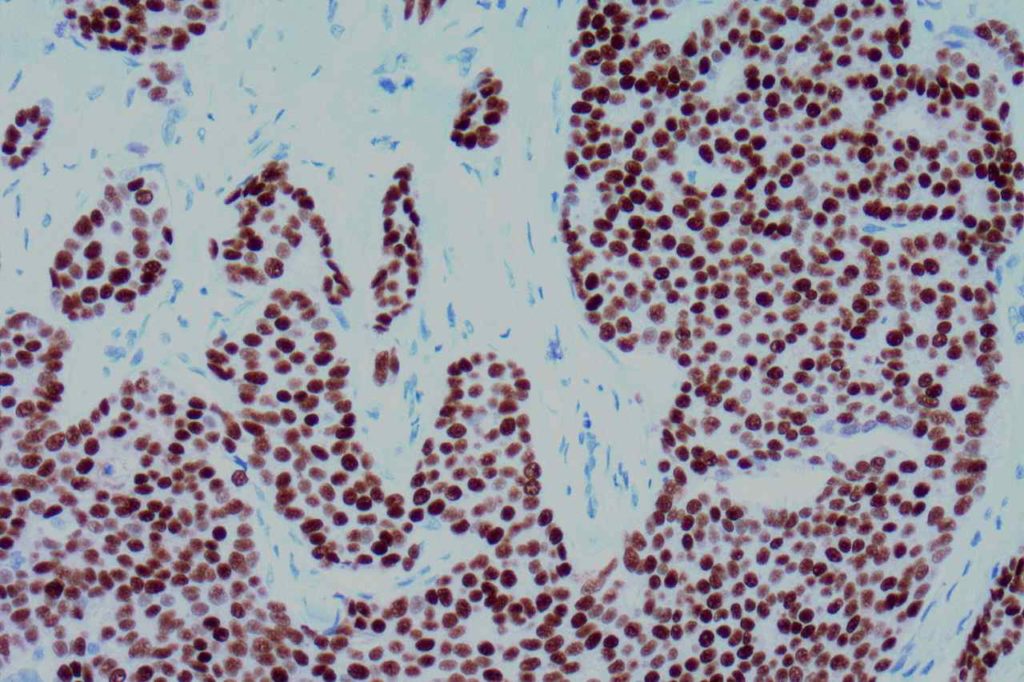
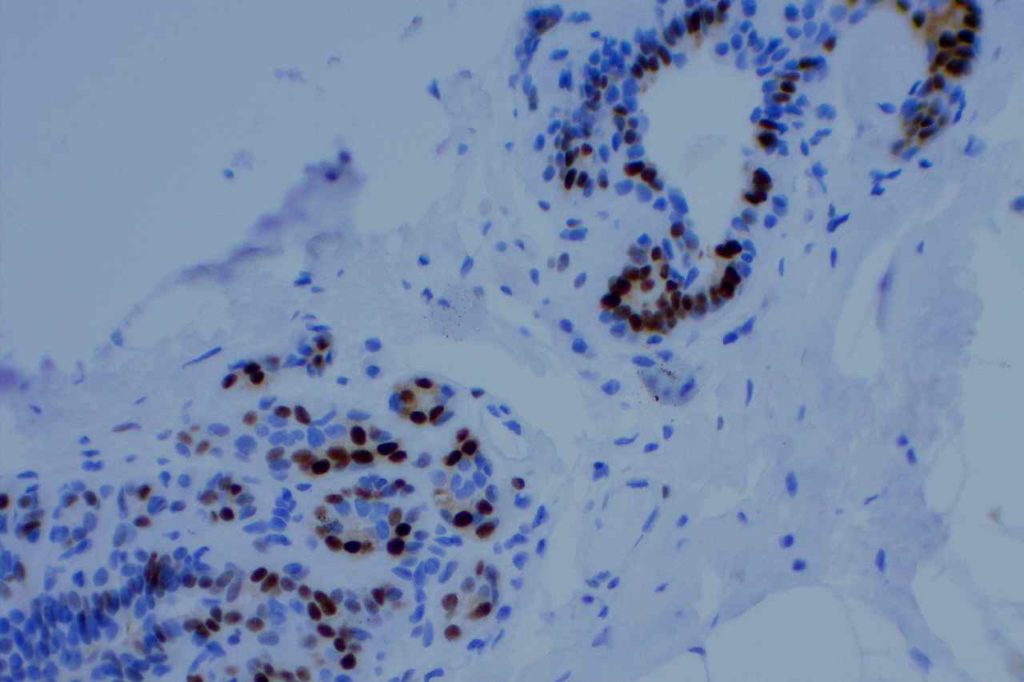
Estrogen Receptor (ER) is a nuclear marker, which is most commonly used to identify breast carcinomas that may be responsive hormonal therapy (e.g. Tamoxifen). It also conveys prognostic information (ER+ has a more favorable prognosis). In normal breast duct epithelium ER will show variable patchy expression, but does not normally stain myoepithelial cells.
Interpretation
Interpretation of ER expression in breast carcinoma is based on percentage positive (+), and stain intensity (1+, 2+, 3+). Benign breast duct epithelium in the background serves as an internal barometer for stain intensity. Breast tumors are considered positive if 1% or more of tumor cells express ER. An important side note: if a case is negative for ER expression, and there is NO INTERNAL CONTROL (i.e. benign duct epithelium), which is positive, then repeat testing on the excision specimen is recommended. Block selection for testing should include benign background tissue, which can serve as an internal control.
In a breast case, if PR is positive and ER is negative, then the ER assay may not be working or the slides have been switched. PR expression should, practically, only occur in the setting of ER expression.
Marker Specificity
Sometimes ER is utilized as a marker to resolve the primary origin of an adenocarinoma. ER is relatively specific for breast origin, but far from perfect. Any “hormonally driven organ” (i.e. ovary, breast, uterus) may commonly show ER expression. Less commonly, practically any organ may show ER expression.
ER is often used as part of a panel to differentiate an endocervical adenocarcinoma (ER negative) from an endometrial adenocarcinoma (ER positive).
Common expression patterns in carcinoma (Dennis, JL, et al)
|
Tumor
|
Expression (%)
|
|
Breast
|
30-60%
|
|
Colon
|
<5%
|
|
Lung
|
<10%
|
|
Ovary
|
10-50%
|
|
Pancreas
|
0%
|
|
Stomach
|
<5%
|
|
Prostate
|
~10%
|
Microscopic Images
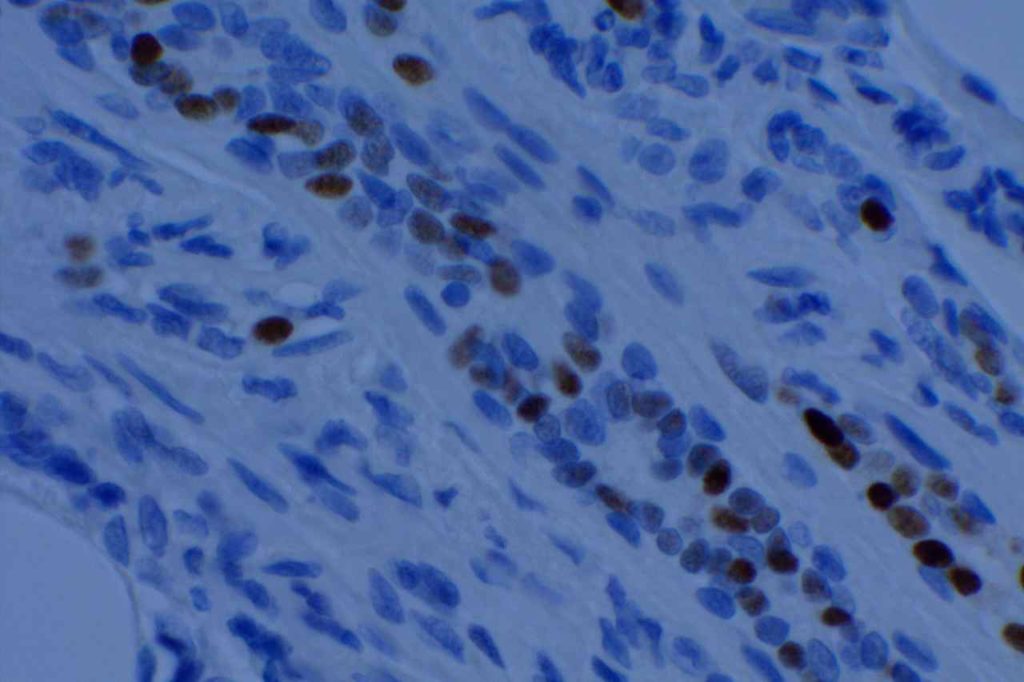
References
Yaziji, H., Taylor, C. R., Goldstein, N. S., Dabbs, D. J., Hammond, E. H., Hewlett, B., et al. (2008). Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Applied Immunohistochemistry & Molecular Morphology : AIMM / Official Publication of the Society for Applied Immunohistochemistry, 16(6), 513–520. doi:10.1097/PAI.0b013e31818a9d3a
Hammond, M. E. H., Hayes, D. F., Dowsett, M., Allred, D. C., Hagerty, K. L., Badve, S., et al. (2010). American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Archives of Pathology & Laboratory Medicine, 134(7), e48–72.
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236
EMA (MUC-1)
Epithelial Membrane Antigen (EMA), originally described in 1979, targets a complex membrane glycoprotein originally isolated from milk fat globules. It is also known as MUC-1. EMA is helpful in identifying epithelial differentiation, but is not entirely specific. In general EMA expression is similar to cytokeratin expression, although hepatocellular carcinomas, adrenocortical neoplasms, and germ cell neoplasms do not express EMA. Some non-epithelial processes, such s ALCL, plasmacytomas, epithelioid sarcoma, synovial sarcoma, meningioma, and T-cell lymphomas may show at least focal positivity. (Wick, M.R.)
In a recent study by Minato, H. et al, EMA was found to be expressed in 79% (n=34) of cases of mesothelioma compared to 13% (n=40) of cases of reactive mesothelial cells.
Photomicrographs
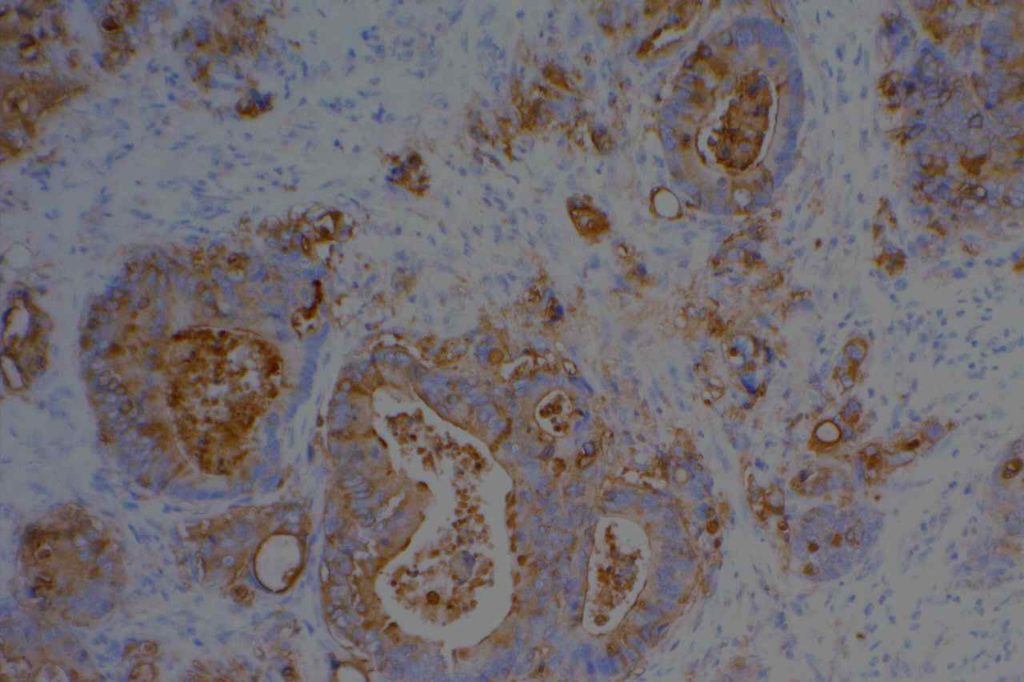
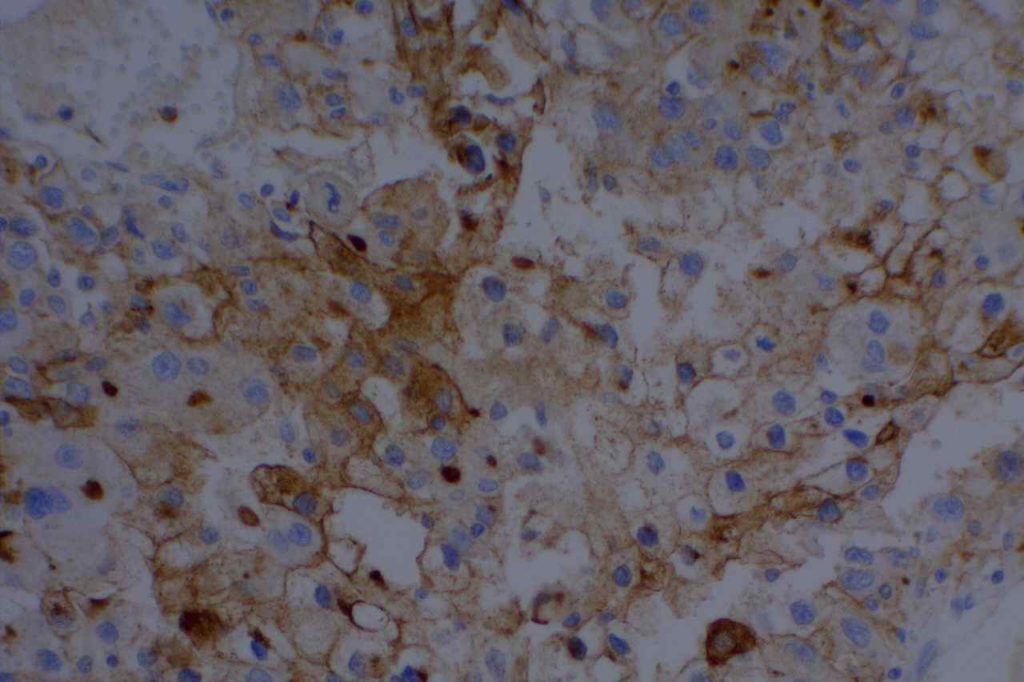
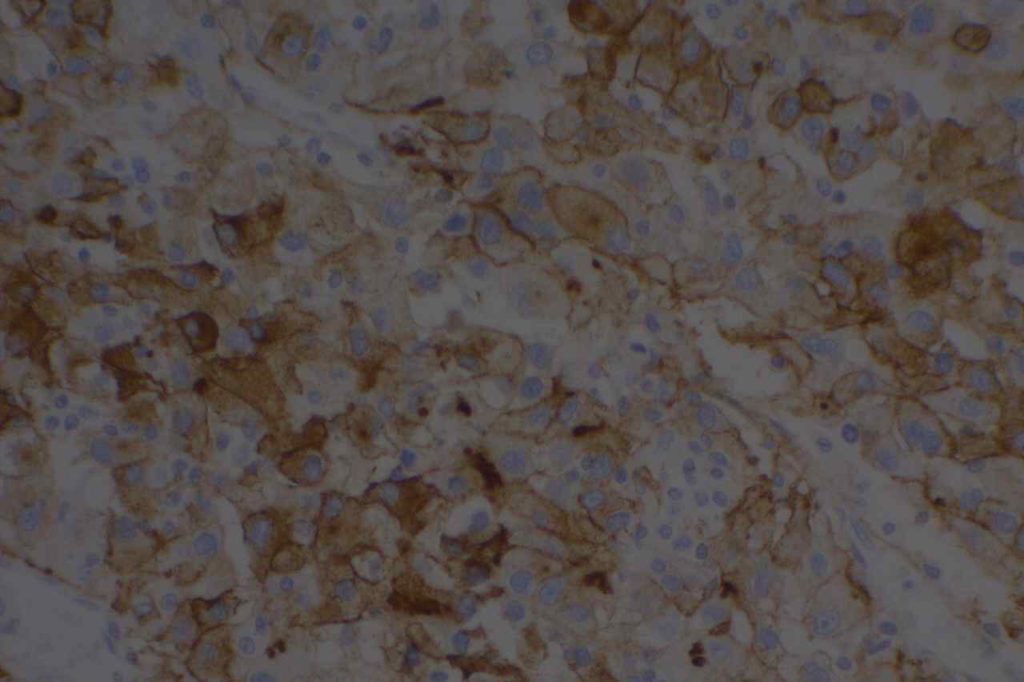
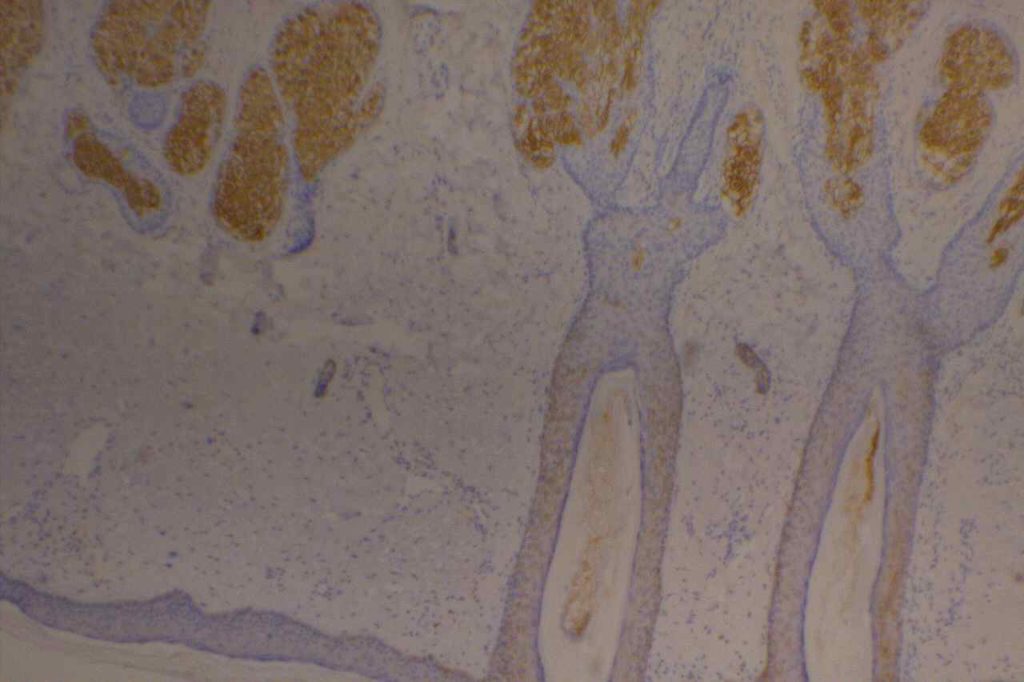
References
Wick, M. R. (2008). Immunohistochemical approaches to the diagnosis of undifferentiated malignant tumors. Annals of Diagnostic Pathology, 12(1), 72–84. doi:10.1016/j.anndiagpath.2007.10.003
Minato, H., Kurose, N., Fukushima, M., Nojima, T., Usuda, K., Sagawa, M., et al. (2014). Comparative Immunohistochemical Analysis of IMP3, GLUT1, EMA, CD146, and Desmin for Distinguishing Malignant Mesothelioma From Reactive Mesothelial Cells. American Journal of Clinical Pathology, 141(1), 85–93. doi:10.1309/AJCP5KNL7QTELLYI
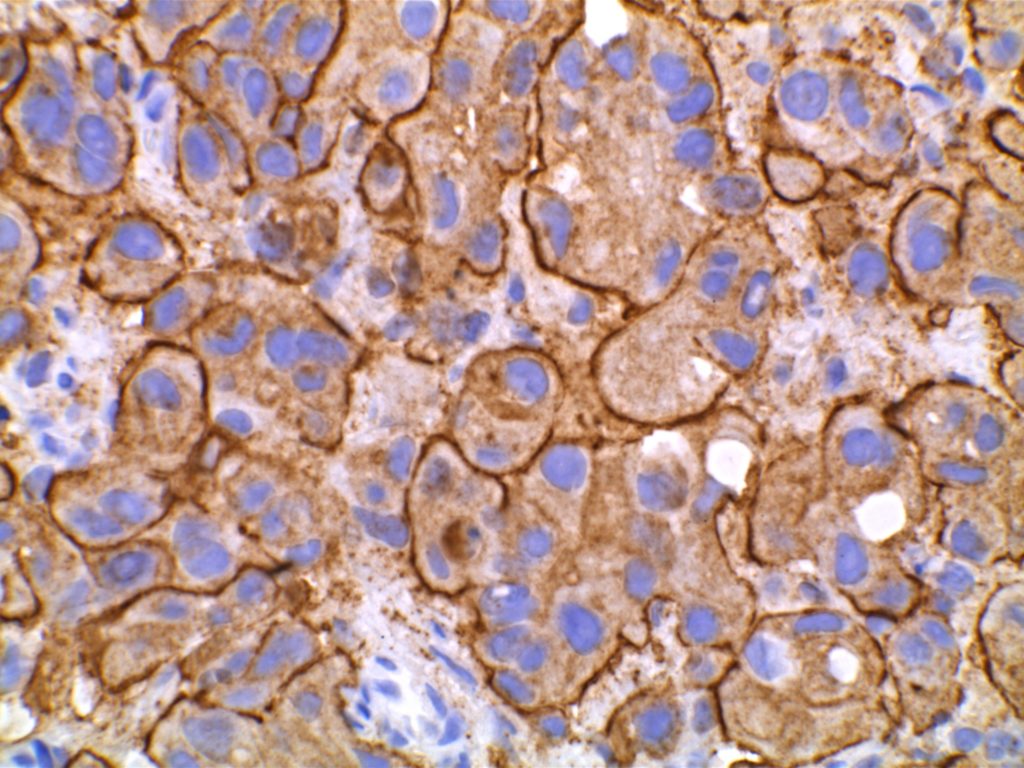
E-Cadherin
E-cadherin is an intercellular adhesion marker, and has a membraneous expression pattern. Its most common use is to help differentiate between DCIS (positive) and LCIS (negative). It is less helpful in differentiating invasive ductal from invasive lobular, because invasive ductal carcinoma will not infrequently lose E-cadherin expression.
If E-cadherin is positive in an invasive breast carcinoma, that would support ductal origin, but negative staining is not helpful / diagnostic between a ductal or lobular process. Practically, if one is having difficulty differentiating an invasive lobular vs. invasive ductal, it is possible that E-cadherin would be negative as the cells become dis-cohesive and lose the intercellular adhesion complexes.
Up to 15% of lobular lesions may exhibit some membraneous E-cadherin expression. In such equivocal cases, p120 catenin or Beta-catenin may be helpful adjunct stains to help resolve discordance between morphology and E-cadherin expression. As is the case with immunohistochemistry in general, a well devised panel of antibodies for a given differential diagnosis is often the best practice, when results or the diagnosis is not straight forward.
DCIS vs. LCIS
|
IHC Stain
|
DCIS
|
LCIS
|
|
E-Cadherin
|
+
|
=
|
|
34BetaE12
|
=
|
+
|
Brattauer, 2002. S. Schnitt, UAMS Lecture, 2002.
Hematopathology
E-cadherin is a sensitive and specific marker for immature erythroid cells. It may be useful (similar to CD71) in identifying immature erythroid precursors, which may be difficult to differentiate from myeloblasts in certain circumstances. CD34 and CD117 (blast markers) may be combined with CD71 and E-cadherin (erythroid markers) to achieve this goal.
Other
E-cadheirn expression has also been studied in numerous other organ tissues individually (e.g. chromophobe renal cell carcinoma) and as part of panels. Use of this stain in close conjunction with current medical literature is recommended.
Photomicrographs
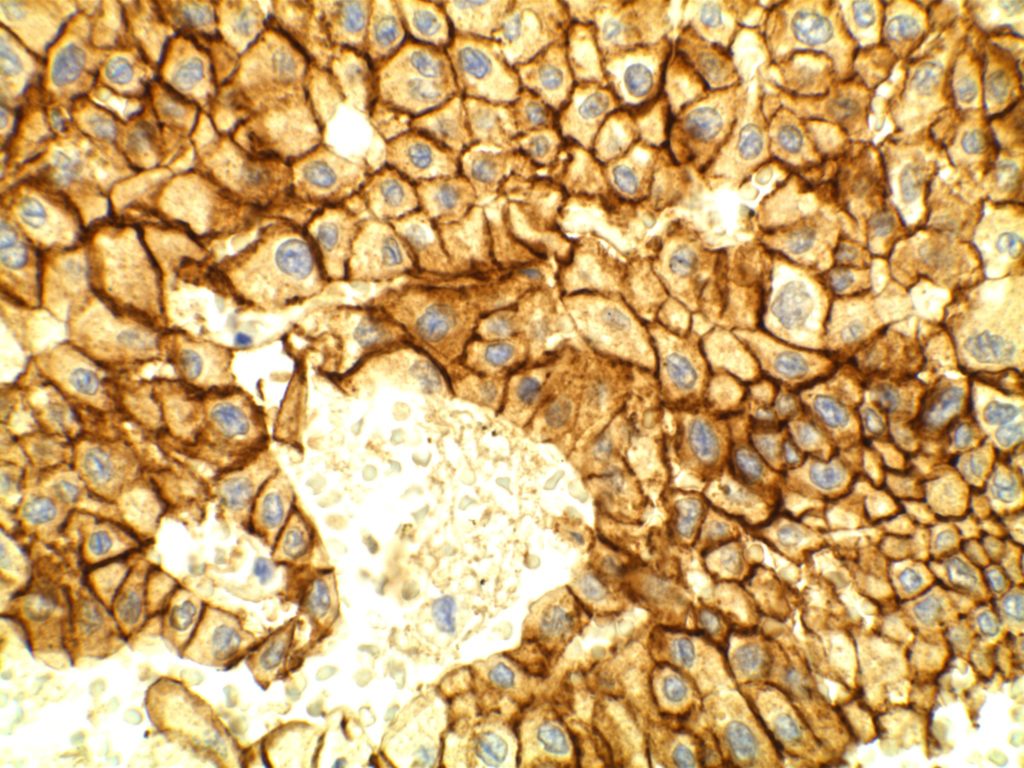
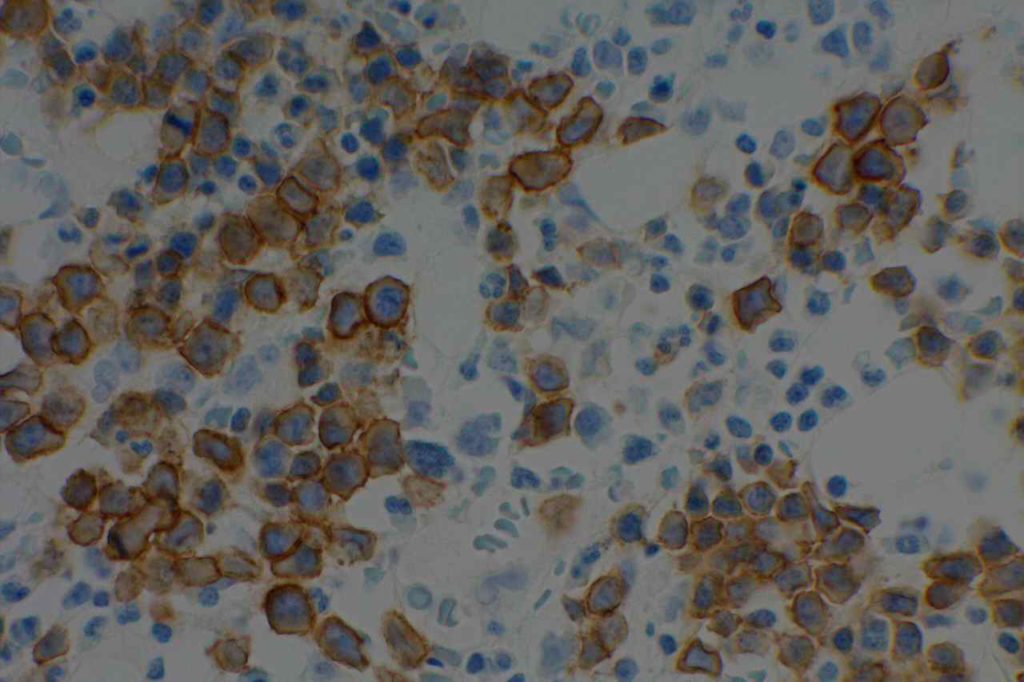
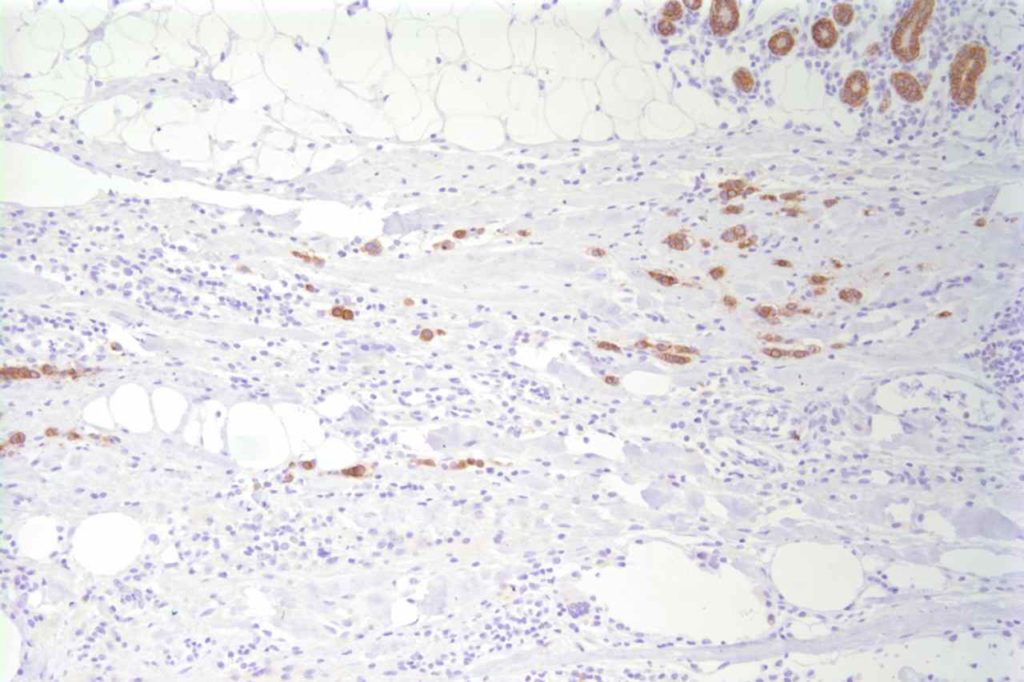
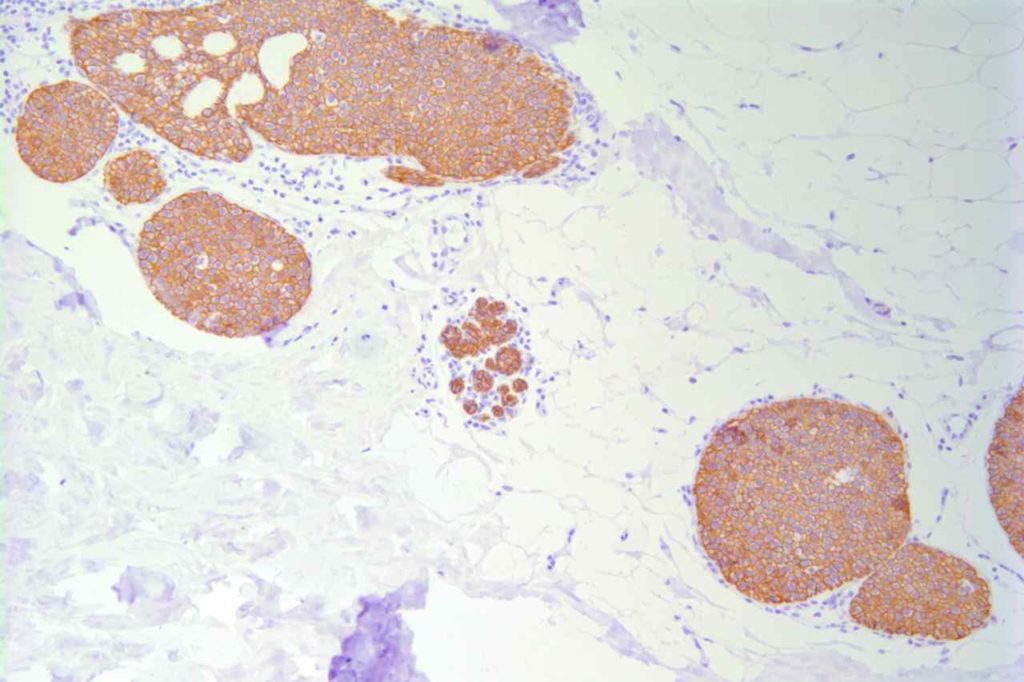
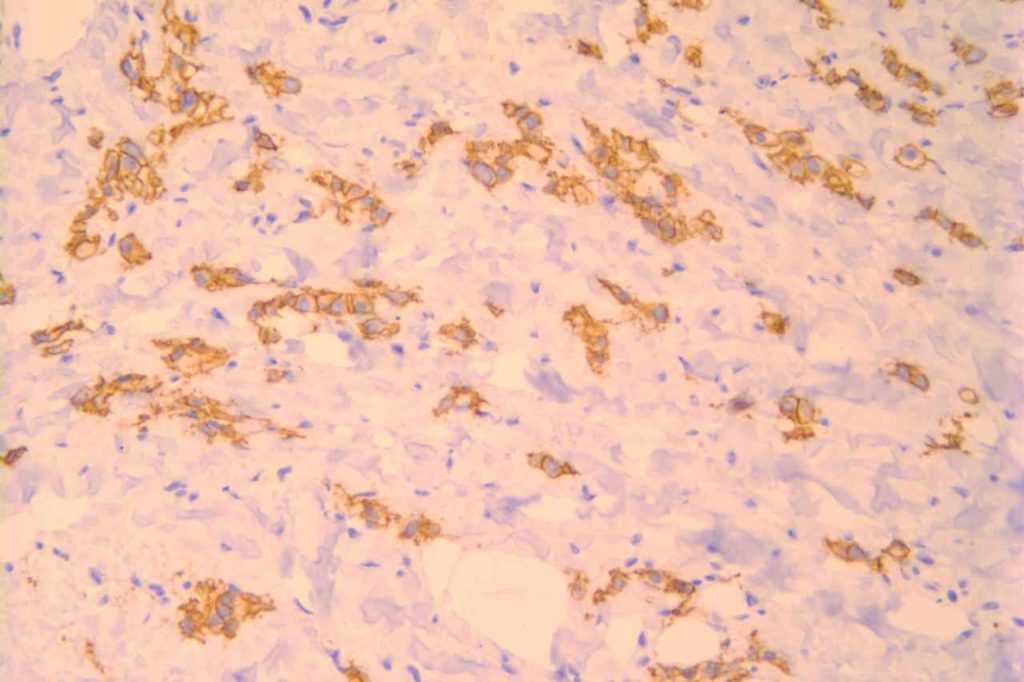
References
Modern Pathology 24, 375-383 (March 2011)
Dabbs, D. J., Schnitt, S. J., Geyer, F. C., Weigelt, B., Baehner, F. L., Decker, T., et al. (2013). Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. The American Journal of Surgical Pathology, 37(7), e1–11. doi:10.1097/PAS.0b013e3182918a2b
Klein, G. E-Cadherin Is Functionally Involved in the Maturationof the Erythroid Lineage. The Journal of Cell Biology, October 1, 1995. pp. 1–7.
Ohgami, R. S., Chisholm, K. M., Ma, L., & Arber, D. A. (2014). E-Cadherin Is a Specific Marker for Erythroid Differentiation and Has Utility, in Combination With CD117 and CD34, for Enumerating Myeloblasts in Hematopoietic Neoplasms. American Journal of Clinical Pathology, 141(5), 656–664. doi:10.1309/AJCP8M4QQTAZPGRP
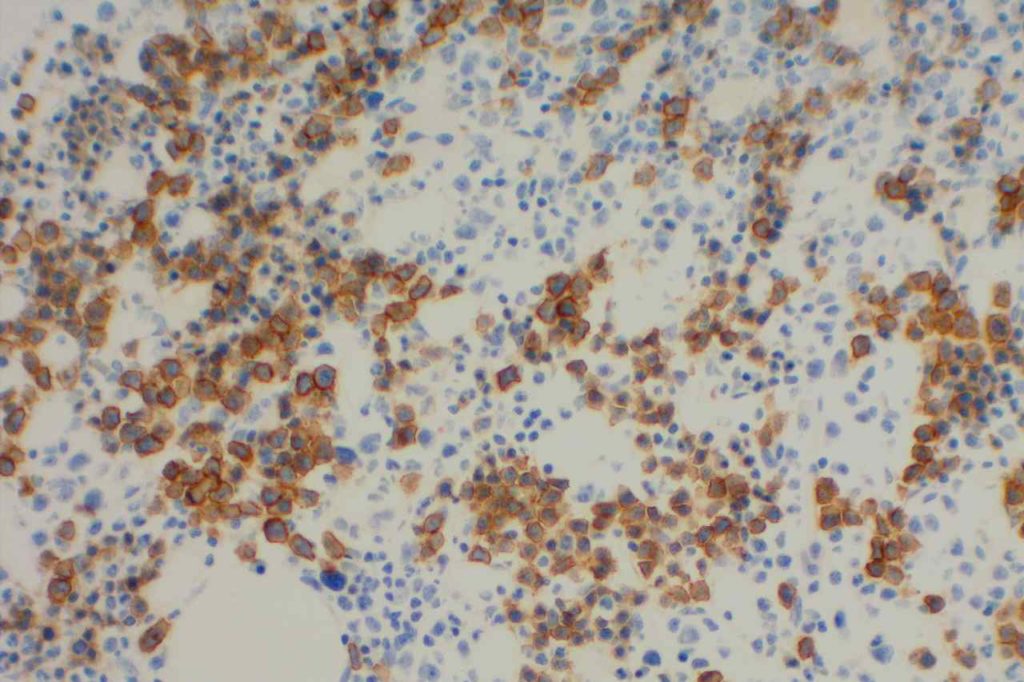
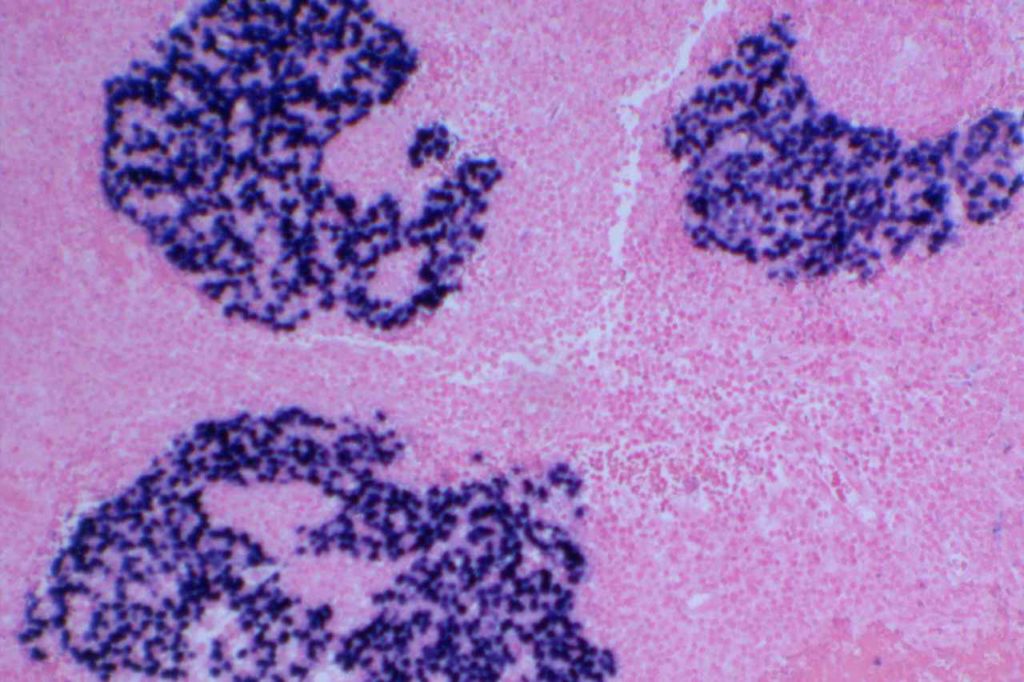
EBV (EBER)
Detection of EBV virus may be performed as stains in tissue sections in one of two ways: (1) EBV IHC or (2) EBER (EBV-encoded RNA) ISH. EBV IHC antibody reacts with the BNLF1 gene product that forms the latent membrane protein (LMP). This marker has limited sensitivity in the 30% range.
EBER expression is localized to the nucleus, while the IHC LMP stains the surface membrane.
Pitfalls
EBER expression can identify lymphocytes latently infected with EBV. Therefore, in CHL for example, the tumor cells must show expression for the case to be considered EBV-related. On the other hand IHC stains for LMP rarely mark latently infected cells in the background, but may show false positivity in poorly fixed tissue, cells in the nervous system, and some uninfected hematopoietic elements (eosinophils and plasma cells). False negative results are more common with IHC LMP in decalcified tissues.
Inter-observer agreement is greater for the interpretation of EBER compared to LMP.
EBV Expression Profile
- DLBCL – EBV is identified in cases of EBV+ DLBCL of the elderly.
- Hodgkin Lymphoma – Approximately 40% of Hodgkin lymphoma cases express EBV in the Hodgkin cells. (Mixed cellularity HL is ~70%+)
- Burkitt Lymphoma – Endemic form
- Nasopharyngeal Carcinoma
- Infectious Mononucleosis
Photomicrographs
References
American Journal of Clinical Pathology. 2002;117(2)
DOG-1
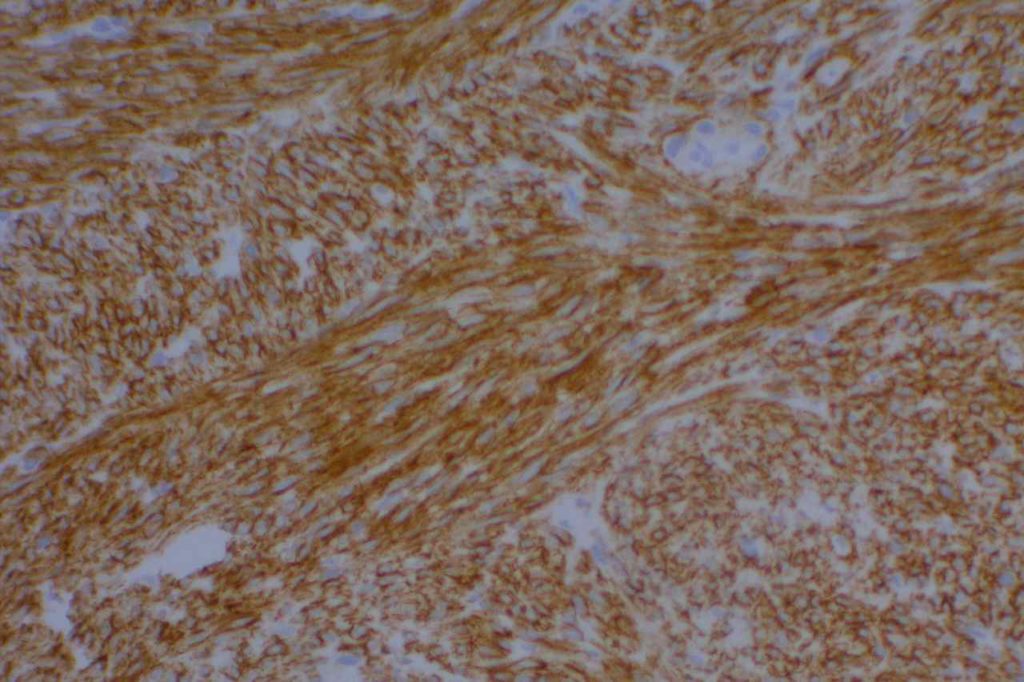
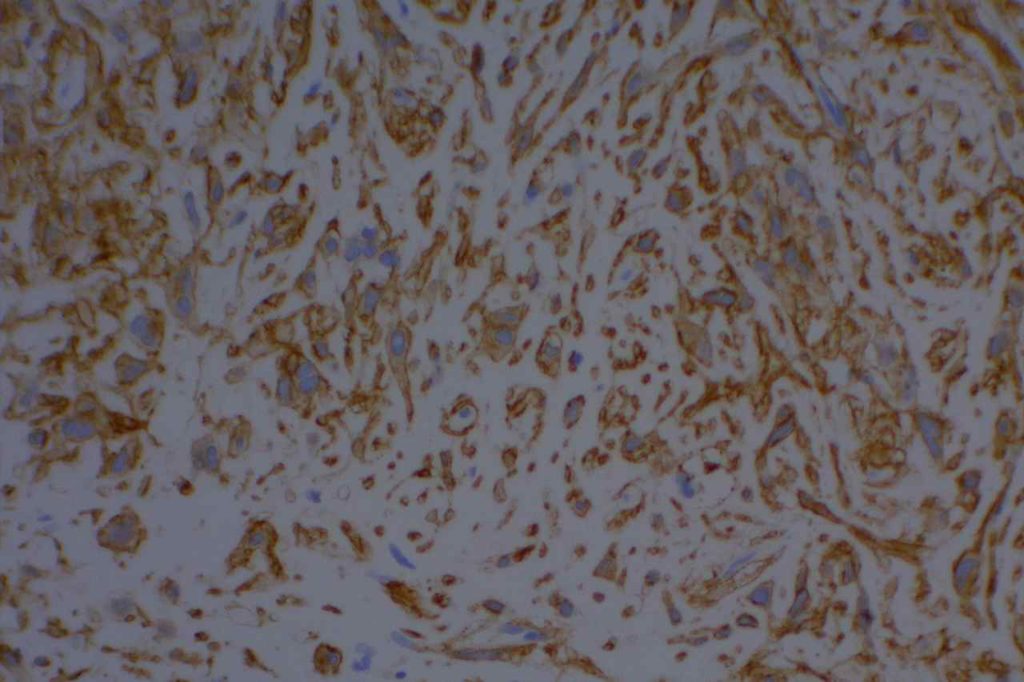
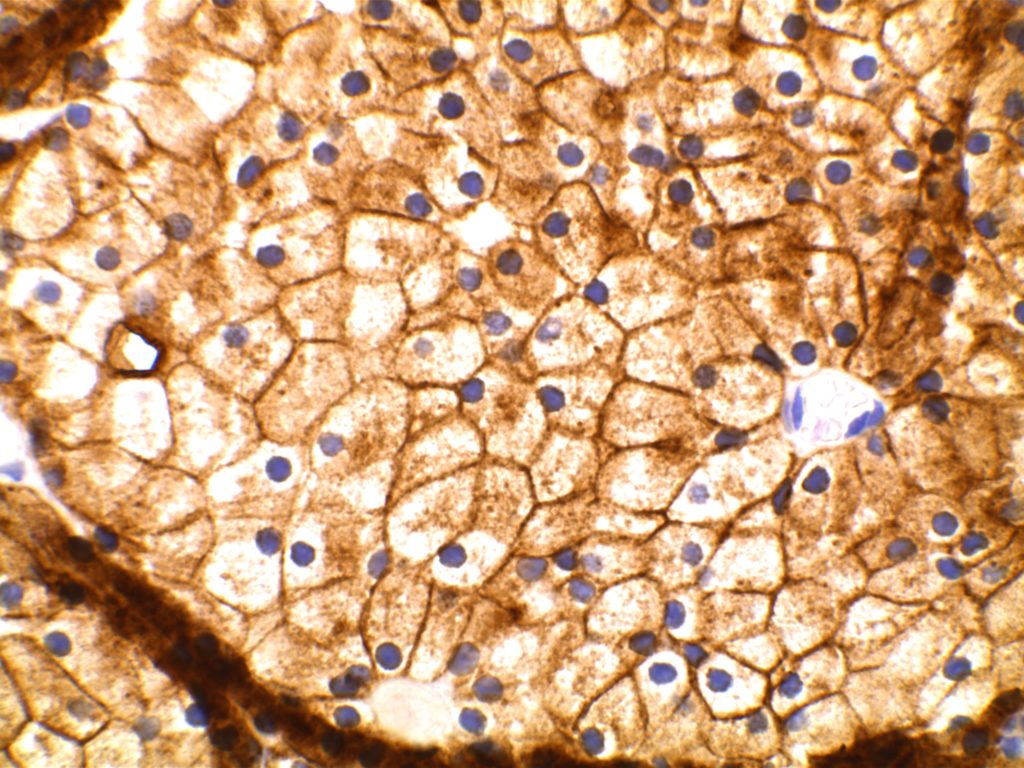
General
DOG-1 is a calcium-activated chloride channel protein (a.k.a. anoctamin-1 or TMEM16a), and has been found to be ubiquitously expressed in GI stromal tumors irrespective of KIT or PDGFRA mutation status. This antibody appears to stain a significant subset of CD117 (c-kit) negative cases (36-46%). Approximately 50% of DOG-1 negative GISTs will express CD117. In the setting of a GIST differential diagnosis, DOG-1 appears to have specificity comparable to CD117. There appears to be expression on a small subset of synovial sarcomas. Normal staining by DOG-1 can be found in interstitial cells of Cajal. Membraneous and apical staining / reactivity may be seen with DOG-1 in non-GIST tumors / cells.
In addition to normal expression in interstitial cells of Cajal in the GI tract, luminal expression can be seen in the gastric mucosa, pancreatic centroacinar cells, intrahepatic bile duct epithelium, bladder, and gallbladder epithelium. Acinic cells in the salivary gland express DOG-1 (cytoplasmic), and may be helpful in confirming the diagnosis of an acinic cell carcinoma.
Clone: K9 (no joke)
Titer: 1:400
Photomicrographs
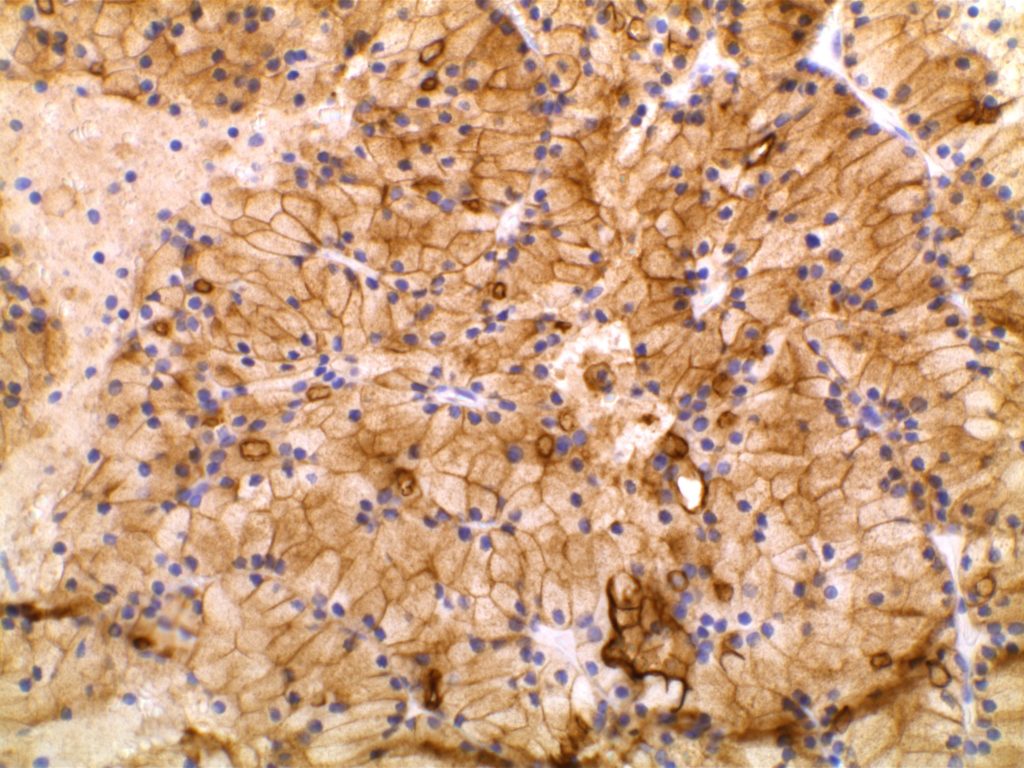
References
Hadi Yaziji, AIMM Annual Meeting, “New Antibodies in Diagnostic Immunohistochemistry”, presentation, 2010.
West RB, et al. American Journal of Pathology. Vol. 165, No. 1, July, 2004.
Chan, J. K. C. (2013). Newly Available Antibodies With Practical Applications in Surgical Pathology. International Journal of Surgical Pathology, 21(6), 553–572. doi:10.1177/1066896913507601
Liegl-Atzwanger, B., Fletcher, J. A., & Fletcher, C. D. M. (2010). Gastrointestinal stromal tumors. Virchows Archiv : an International Journal of Pathology. doi:10.1007/s00428-010-0891-y
Patil, D. T., & Rubin, B. P. (2011). Gastrointestinal stromal tumor: advances in diagnosis and management. Archives of Pathology & Laboratory Medicine, 135(10), 1298–1310. doi:10.5858/arpa.2011-0022-RA
Chan JKC. Newly Available Antibodies With Practical Applications in Surgical Pathology. International Journal of Surgical Pathology. 2013;21: 553–572. doi:10.1177/1066896913507601
D2-40
D2-40 is a marker for a sialoglycoprotein found on lymphatic endothelium. This marker is most commonly used to identify lymphatic invasion, and can help differentiate between lymphatic or vascular invasion with the additional support of a general endothelial marker (e.g. CD31 or CD34), which mark both vascular and lymphatics. It is not recommended to use D2-40 without CD31 and/or CD34 when evaluating for lymphatic invasion. Other cell types (including myoepithelial cells in the breast) can also express D2-40, which may result in misidentification of lymphatic invasion.
In addition expression is also found in germ cell neoplasia and fetal testicular monocytes. (Mohammad and Ellis)
D2-40 will also be positive in mesotheliomas, but negative in adenocarcinomas. Chu et al. showed 96% of epithelioid mesotheliomas and 65% of ovarian serous carcinomas reacted with D2-40 with membraneous staining. Other carcinomas generally did not express D2-40 in their study. There is no evidence that D2-40 is helpful with spindle-cell/sarcomatoid mesotheliomas.
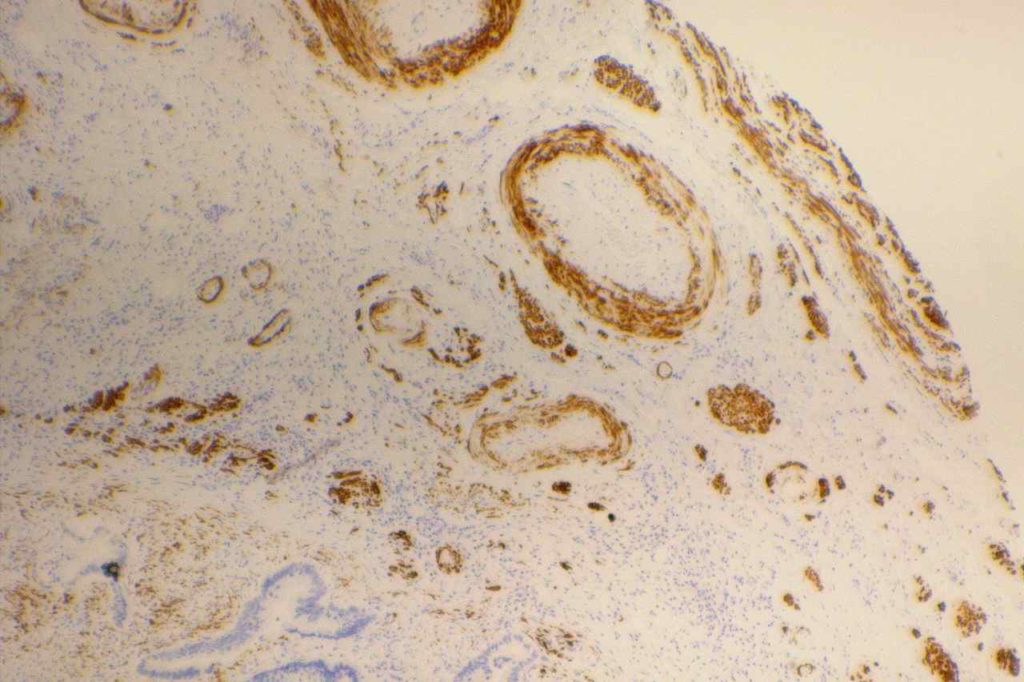
Kaposi Sarcoma
D2-40 is expressed in approximately 2/3rds of cases of Kaposi’s Sarcoma. It stains in a similar pattern as CD31 and CD34.
Germ Cell Tumors
Seminomas show expression for D2-40, along with a subset of embryonic carcinomas. Yolk sac tumors are not know to express D2-40. (Bai, et al.)
Photomicrographs
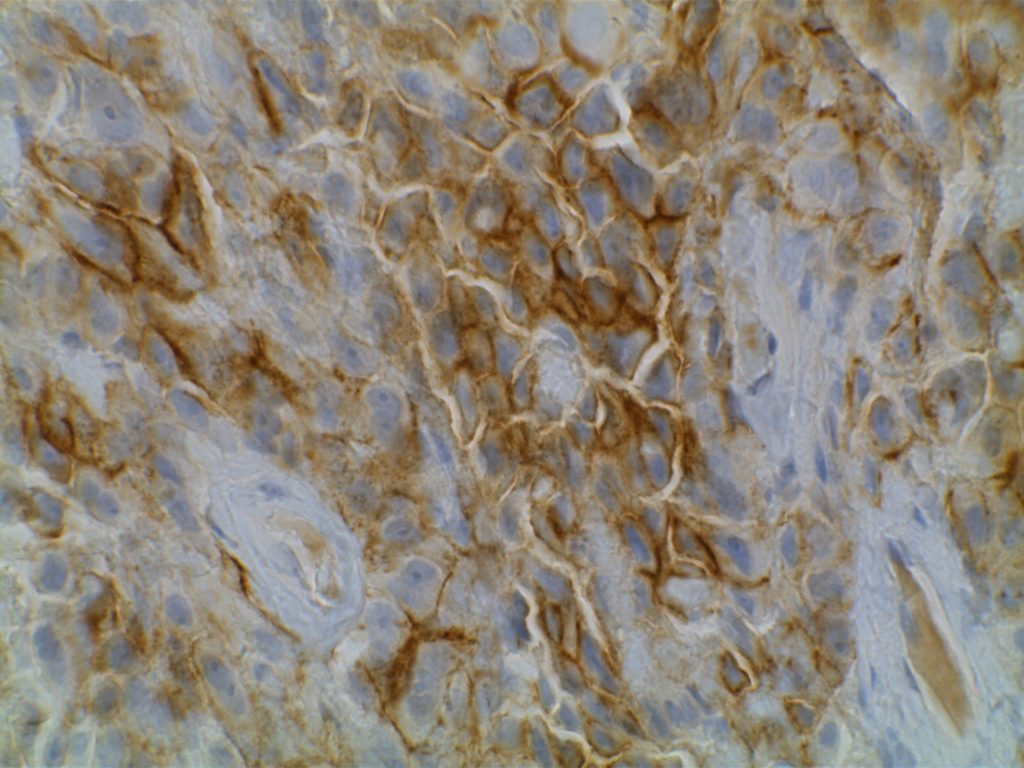
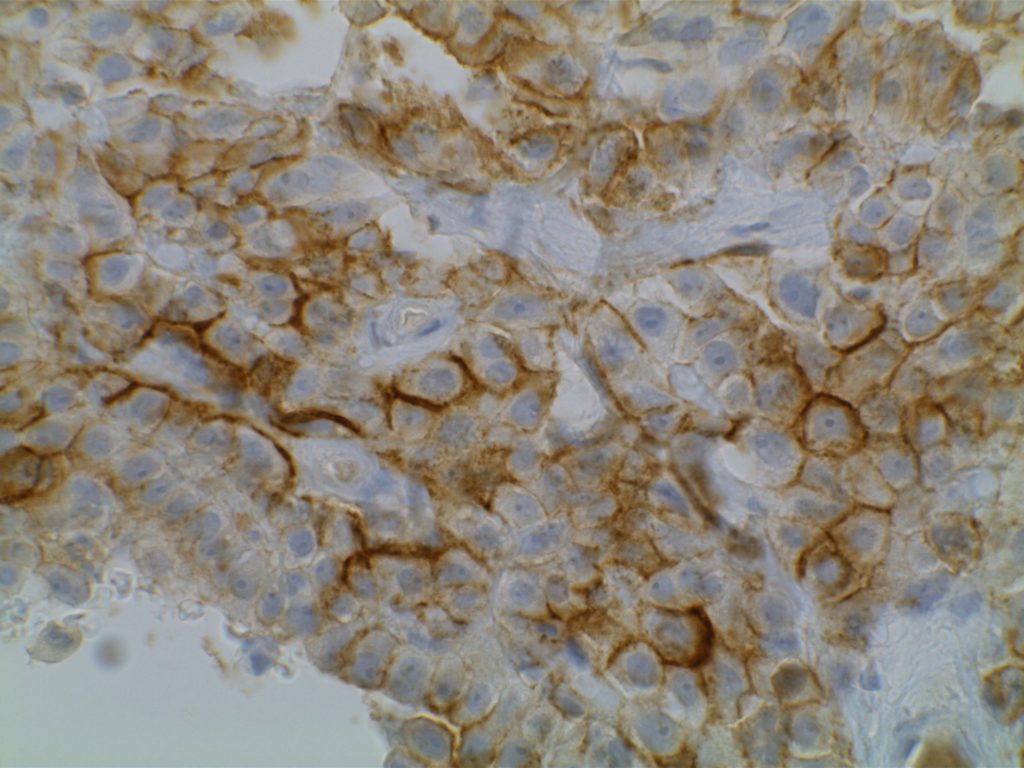
References
Bai S, Wei S, Pasha TL, Yao Y, Tomaszewski JE, Bing Z. Immunohistochemical Studies of Metastatic Germ-Cell Tumors in Retroperitoneal Dissection Specimens: A Sensitive and Specific Panel. International Journal of Surgical Pathology. 2013;21: 342–351. doi:10.1177/1066896912471849
Rosado FGN, Itani DM, Coffin CM, Cates JM. Utility of immunohistochemical staining with FLI1, D2-40, CD31, and CD34 in the diagnosis of acquired immunodeficiency syndrome-related and non-acquired immunodeficiency syndrome-related Kaposi sarcoma. Arch Pathol Lab Med. 2012;136: 301–304. doi:10.5858/arpa.2011-0213-OA
Ordóñez NG. Immunohistochemical diagnosis of epithelioid mesothelioma: an update. Arch Pathol Lab Med. 2005;129: 1407–1414.
Wick MR. Immunohistochemical approaches to the diagnosis of undifferentiated malignant tumors. Annals of Diagnostic Pathology. 2008;12: 72–84. doi:10.1016/j.anndiagpath.2007.10.003
Marchevsky AM. Application of immunohistochemistry to the diagnosis of malignant mesothelioma. Arch Pathol Lab Med. 2008;132: 397–401.
Mohammed RAA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31: 1825–1833. doi:10.1097/PAS.0b013e31806841f6
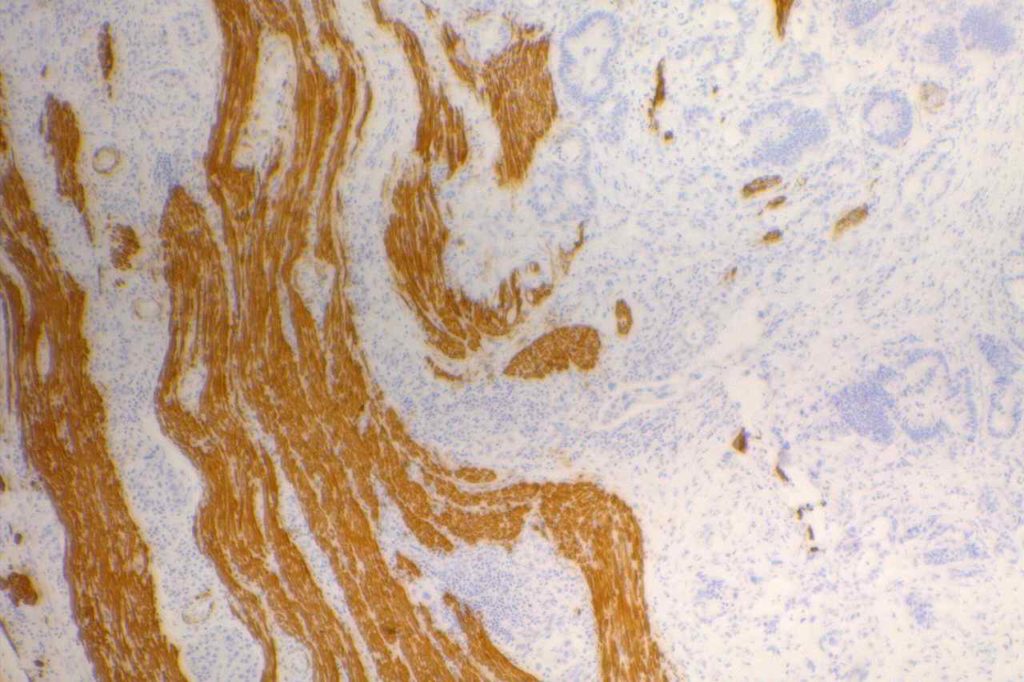
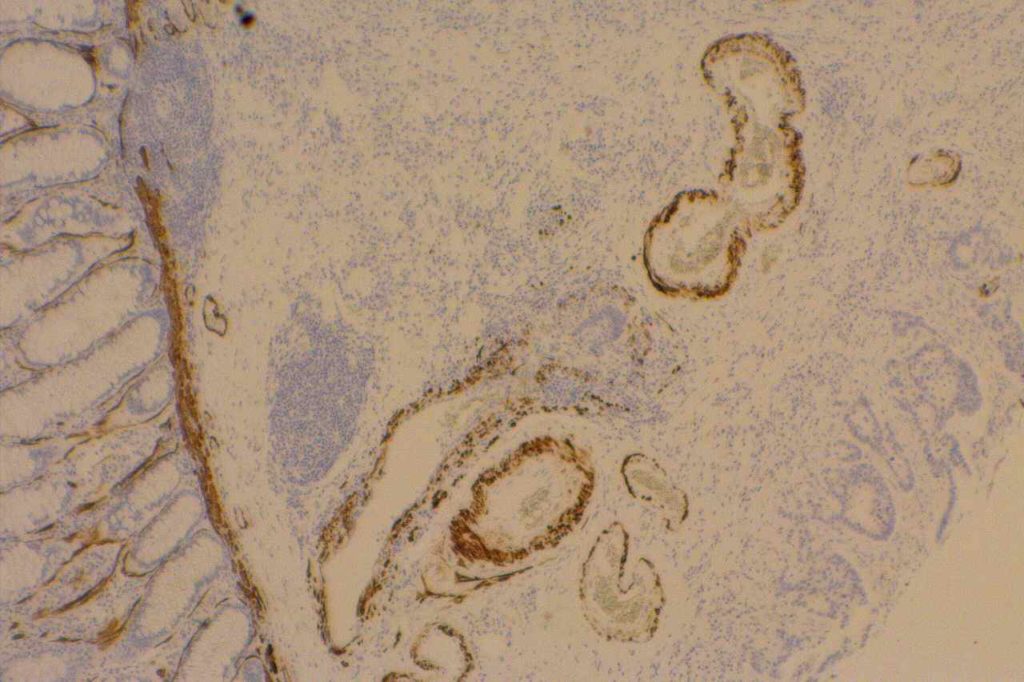
Desmin
Desmin is a muscle marker for intermediate filaments, which are present in smooth and striated muscle. It shows variable myofibroblastic expression. It is most commonly used to identify muscle differentiation in neoplastic processes (e.g. leiomyoma, rhabdomyosarcoma, etc.).
It is interesting that reactive mesothelial cells are often positive for desmin (>50%), compared to mesothelioma (<10%) or carcinoma (<5%).
Photomicrographs
References
Davidson, B., Nielsen, S., Christensen, J., Asschenfeldt, P., Berner, A., Risberg, B., Johansen, P. (2001). The role of desmin and N-cadherin in effusion cytology: a comparative study using established markers of mesothelial and epithelial cells. American Journal of Surgical Pathology, Nov;25(11):1405-12.
Minato, H., Kurose, N., Fukushima, M., Nojima, T., Usuda, K., Sagawa, M., et al. (2014). Comparative Immunohistochemical Analysis of IMP3, GLUT1, EMA, CD146, and Desmin for Distinguishing Malignant Mesothelioma From Reactive Mesothelial Cells. American Journal of Clinical Pathology, 141(1), 85–93. doi:10.1309/AJCP5KNL7QTELLYI
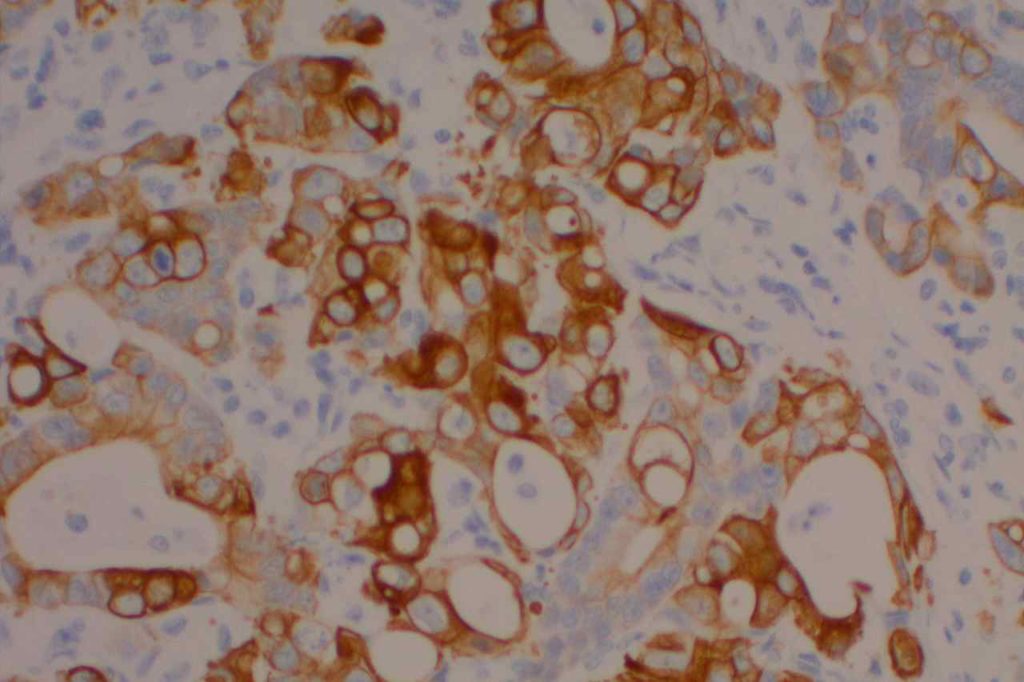
CK20
CK20 is an intermediate filament with a selective expression pattern in different carcinomas, which when combined with CD7 is useful in the work up of carcinomas of unknown primary origin.
Common expression patterns in carcinoma (Dennis, JL, et al).
|
Tumor
|
(%)
|
|
Breast
|
<10%
|
|
Colon
|
>80%
|
|
Lung
|
<10%
|
|
Ovary
|
<10%
|
|
Pancreas
|
35-50%
|
|
Stomach
|
30-50%
|
|
Prostate
|
<10%
|
Moll, RT, et al. Cytokeratin expression in various tumors.
|
Tumor
|
CK8/CK18
|
CK19
|
CK7
|
CK20
|
CK5
|
|
Hepatocellular Ca.
|
+
|
+/-
|
+/-
|
+/-
|
=
|
|
Colorectal ACA
|
+
|
+
|
+/-
|
+
|
=
|
|
Stomach ACA
|
+
|
+
|
+/-
|
+/-
|
=
|
|
Pancreas Ductal ACA
|
+
|
+
|
+
|
+/-
|
+/-
|
|
Lung ACA
|
+
|
+
|
+
|
=
|
=
|
|
Breast Inv. Ductal
|
+
|
+
|
+
|
=
|
+/-
|
|
Endometrium ACA
|
+
|
+
|
+
|
=
|
+/-
|
|
Ovary ACA
|
+
|
+
|
+
|
=
|
=
|
|
RCC, Clear Cell Type
|
+
|
+/-
|
=
|
=
|
=
|
|
RCC, Papillary Type
|
+
|
+
|
+
|
=
|
=
|
|
RCC, Chromophobe
|
+
|
+/-
|
+
|
=
|
=
|
|
Mesothelioma
|
+
|
+
|
+/-
|
=
|
+
|
|
Lung, Small Cell Ca.
|
+
|
+/-
|
=
|
=
|
=
|
|
Merkel Cell Ca.
|
+
|
+
|
=
|
+
|
=
|
|
Urothelial Carcinoma
|
+
|
+
|
+
|
+/-
|
+/-
|
|
Squamous Cell Ca.
|
+/-
|
+/-
|
=
|
=
|
+
|
Key: “+/-“, focal staining in some cases. “=“, negative, “+”, positive.
Photomicrographs
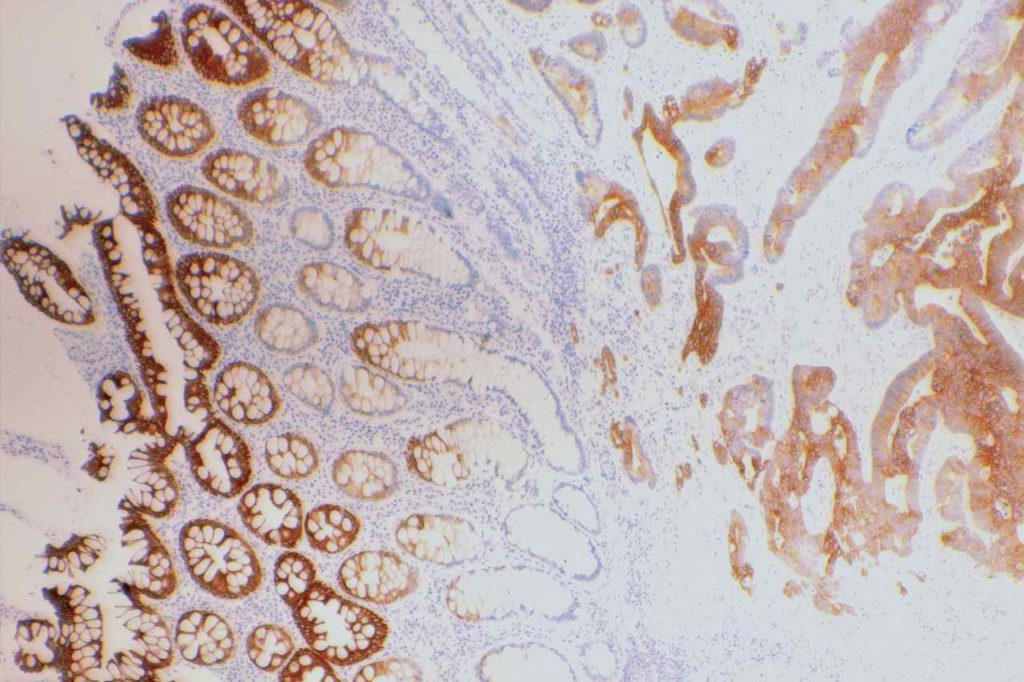
References
Hadi, AIMM Annual Meeting, “The Thirty Most Important Antibodies”, presentation, 2011.
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236
Moll, R., Divo, M., & Langbein, L. (2008). The human keratins: biology and pathology. Histochemistry and Cell Biology, 129(6), 705–733. doi:10.1007/s00418-008-0435-6
CK7
CK7 is an intermediate filament with a selective expression pattern in different carcinomas, which when combined with CD20 is useful in the work up of carcinomas of unknown primary origin.
Common expression patterns in carcinoma (Dennis, JL, et al).
|
Tumor
|
Expression %
|
|
Breast
|
>80%
|
|
Colon
|
<10%
|
|
Lung
|
>90%
|
|
Ovary
|
>80%
|
|
Pancreas
|
>80%
|
|
Stomach
|
35-70%
|
|
Prostate
|
~10%
|
Moll, RT, et al. Cytokeratin expression in various tumors.
|
Tumor
|
CK8/CK18
|
CK19
|
CK7
|
CK20
|
CK5
|
|
Hepatocellular Ca.
|
+
|
+/-
|
+/-
|
+/-
|
=
|
|
Colorectal ACA
|
+
|
+
|
+/-
|
+
|
=
|
|
Stomach ACA
|
+
|
+
|
+/-
|
+/-
|
=
|
|
Pancreas Ductal ACA
|
+
|
+
|
+
|
+/-
|
+/-
|
|
Lung ACA
|
+
|
+
|
+
|
=
|
=
|
|
Breast Inv. Ductal
|
+
|
+
|
+
|
=
|
+/-
|
|
Endometrium ACA
|
+
|
+
|
+
|
=
|
+/-
|
|
Ovary ACA
|
+
|
+
|
+
|
=
|
=
|
|
RCC, Clear Cell Type
|
+
|
+/-
|
=
|
=
|
=
|
|
RCC, Papillary Type
|
+
|
+
|
+
|
=
|
=
|
|
RCC, Chromophobe
|
+
|
+/-
|
+
|
=
|
=
|
|
Mesothelioma
|
+
|
+
|
+/-
|
=
|
+
|
|
Lung, Small Cell Ca.
|
+
|
+/-
|
=
|
=
|
=
|
|
Merkel Cell Ca.
|
+
|
+
|
=
|
+
|
=
|
|
Urothelial Carcinoma
|
+
|
+
|
+
|
+/-
|
+/-
|
|
Squamous Cell Ca.
|
+/-
|
+/-
|
=
|
=
|
+
|
Key: “+/-“, focal staining in some cases. “=“, negative, “+”, positive.
Photomicrographs
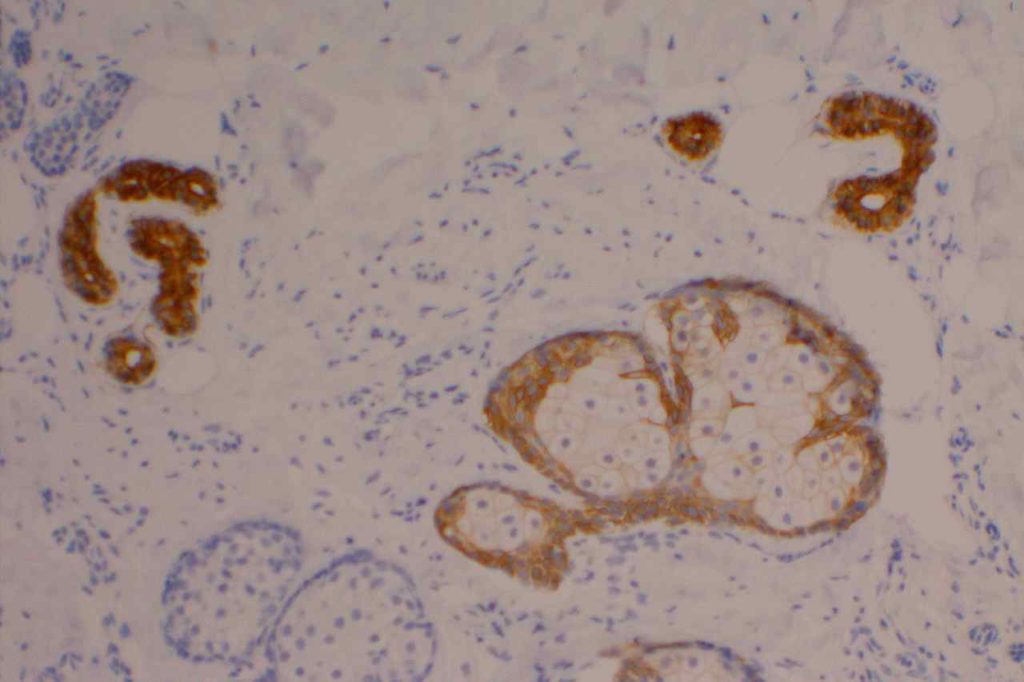
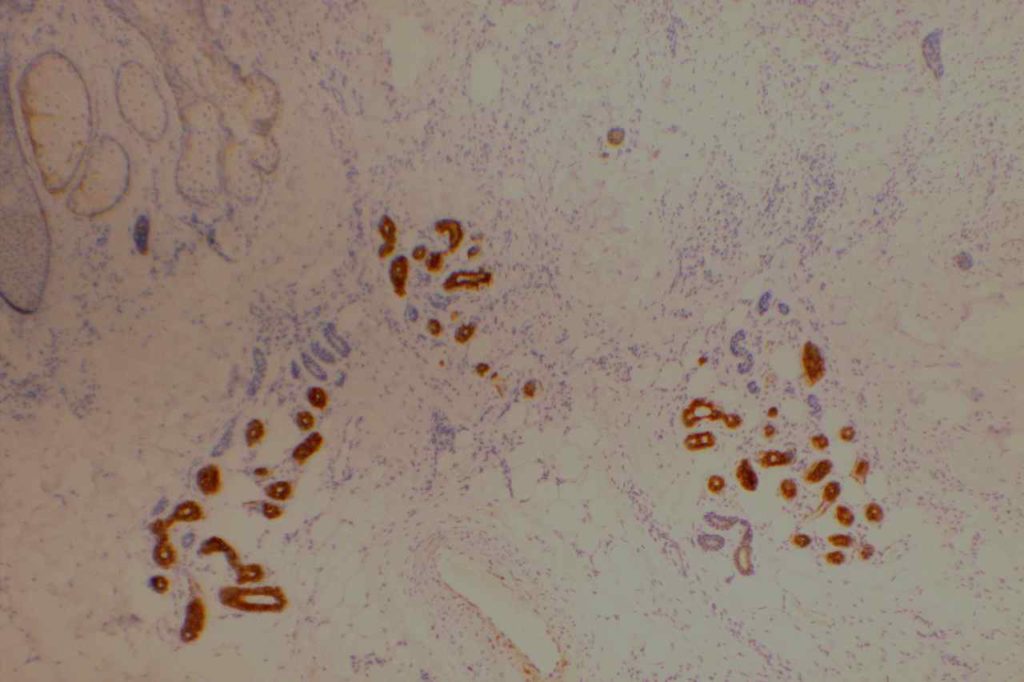
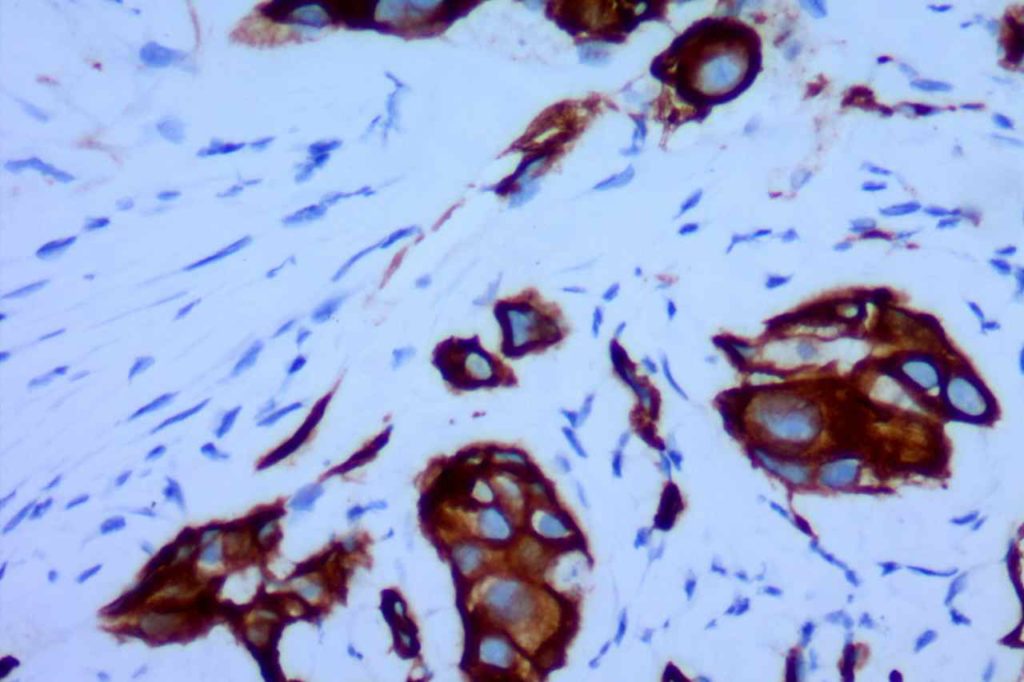
References
Hadi, AIMM Annual Meeting, “The Thirty Most Important Antibodies”, presentation, 2011.
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236
Moll, R., Divo, M., & Langbein, L. (2008). The human keratins: biology and pathology. Histochemistry and Cell Biology, 129(6), 705–733. doi:10.1007/s00418-008-0435-6