Category Archives: G – M Antibodies
Mammaglobin
MART-1/Melan-A
LEF1
MOC-31
MOC-31 is a glycoprotein on the cell-membrane (epithelial glycoprotein 2/epithelial cell adhesion molecule – Ep-CAM) that is widely distributed on epithelial cells and tumor cells. MOC-31 is often used to differentiate adenocarcinoma (93% positive) from mesothelioma (93% negative). Other tumors also typically negative for MOC-31 include: hepatocellular carcinoma (HCC), germ cell tumors, and renal cell (some report up to 50%+) carcinomas. Some tumors may have characteristic staining patterns (e.g. membraneous vs. cytoplasmic or apical).
MUM-1/IRF-4
Lysozyme
References
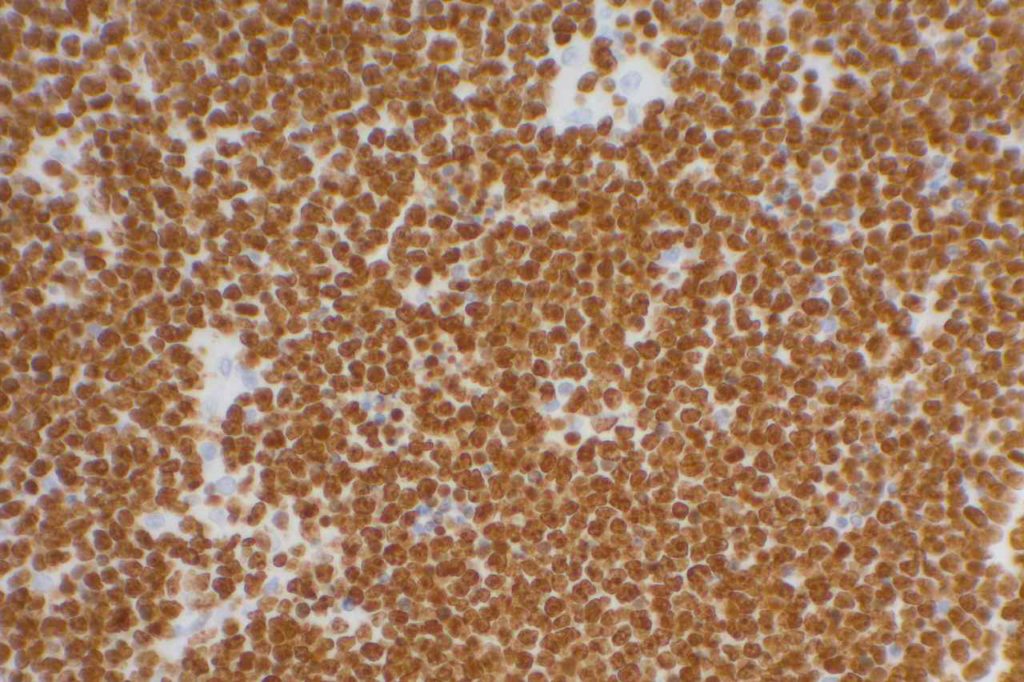
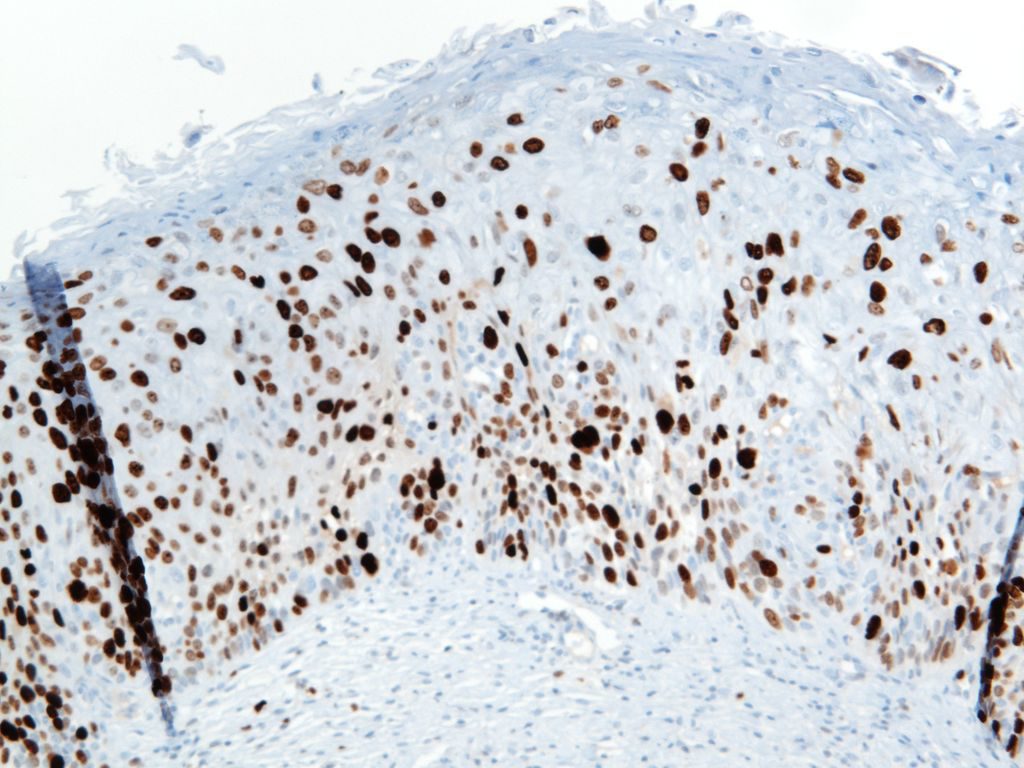
Ki-67
Photomicrographs
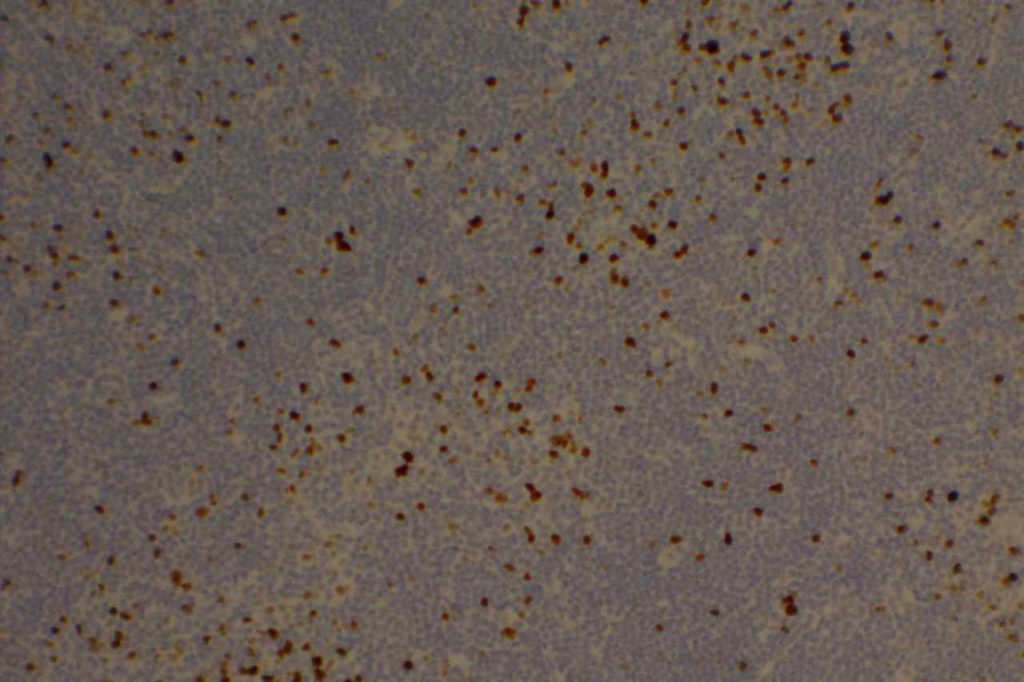
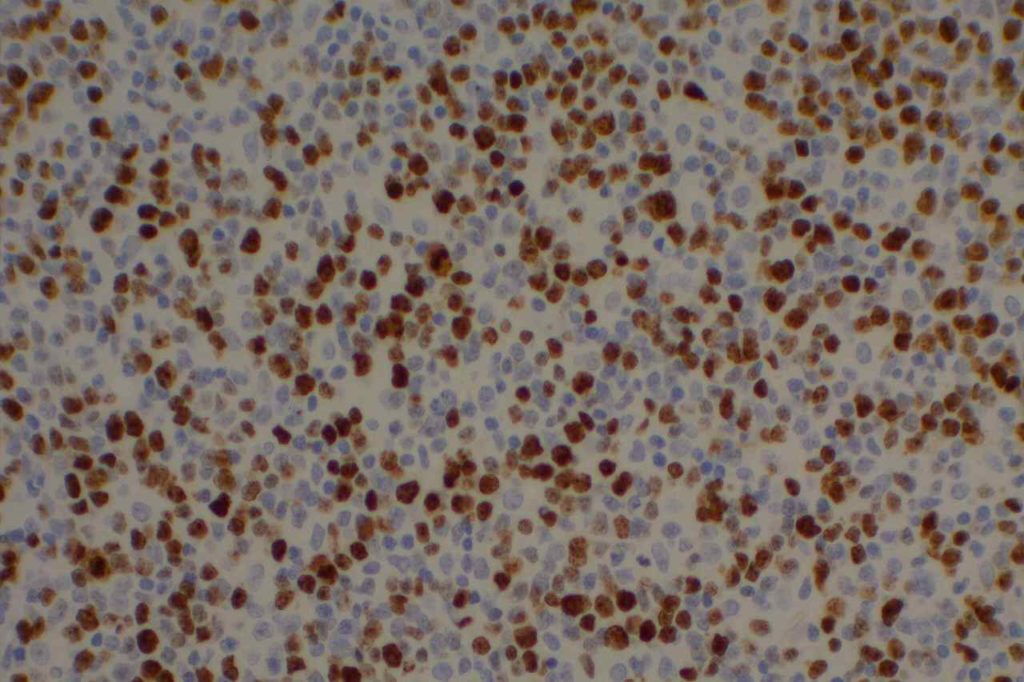
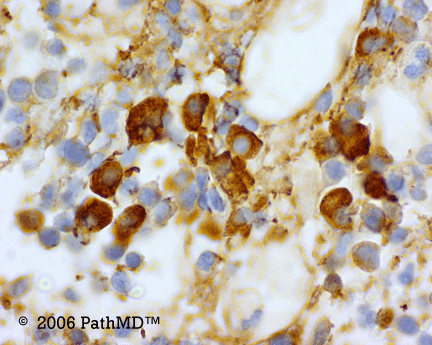
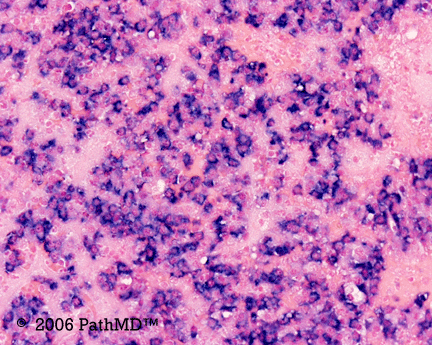
Kappa/Lambda
Photomicrographs
References
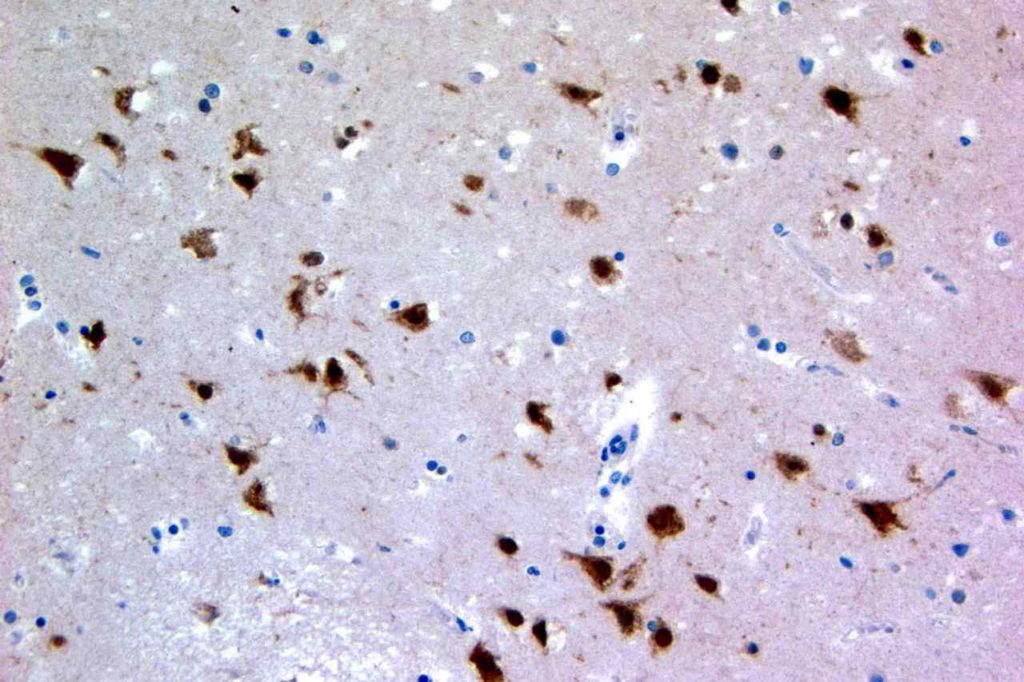
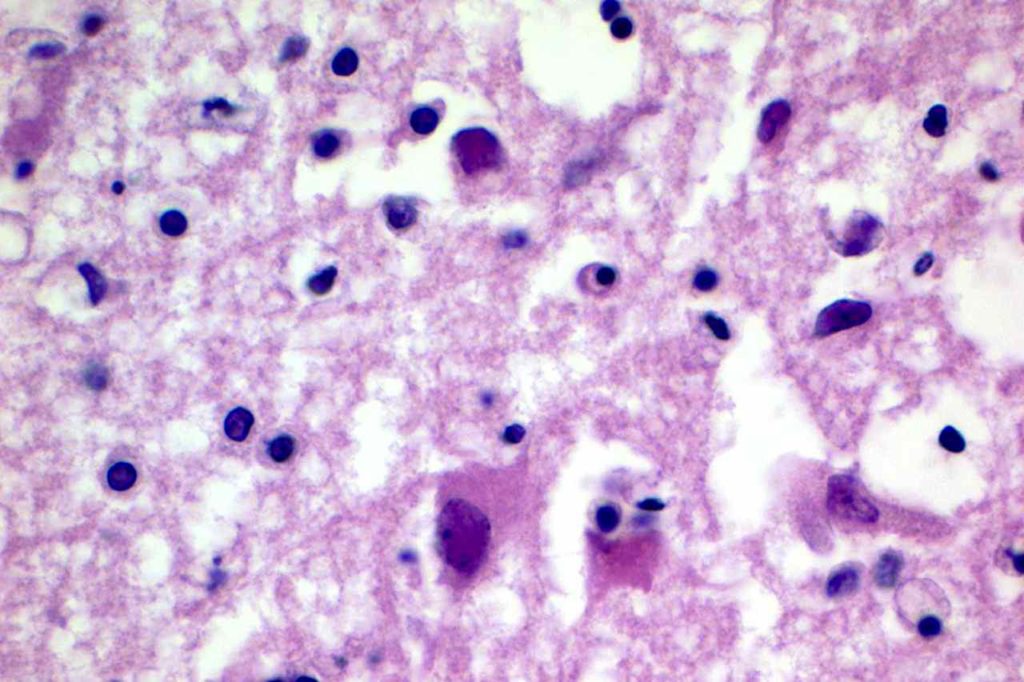
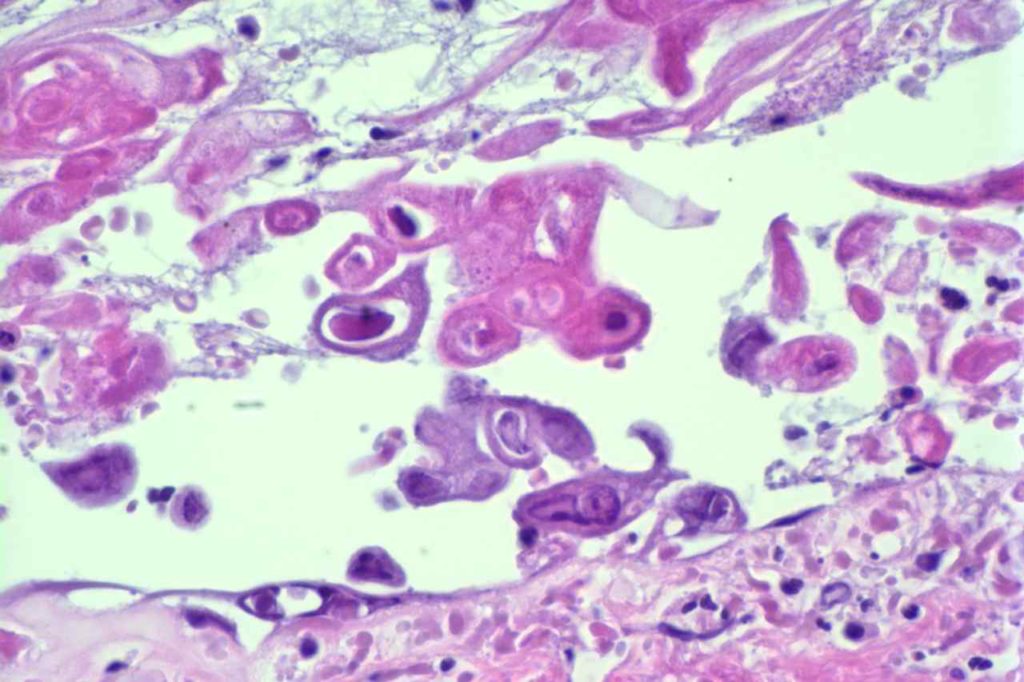
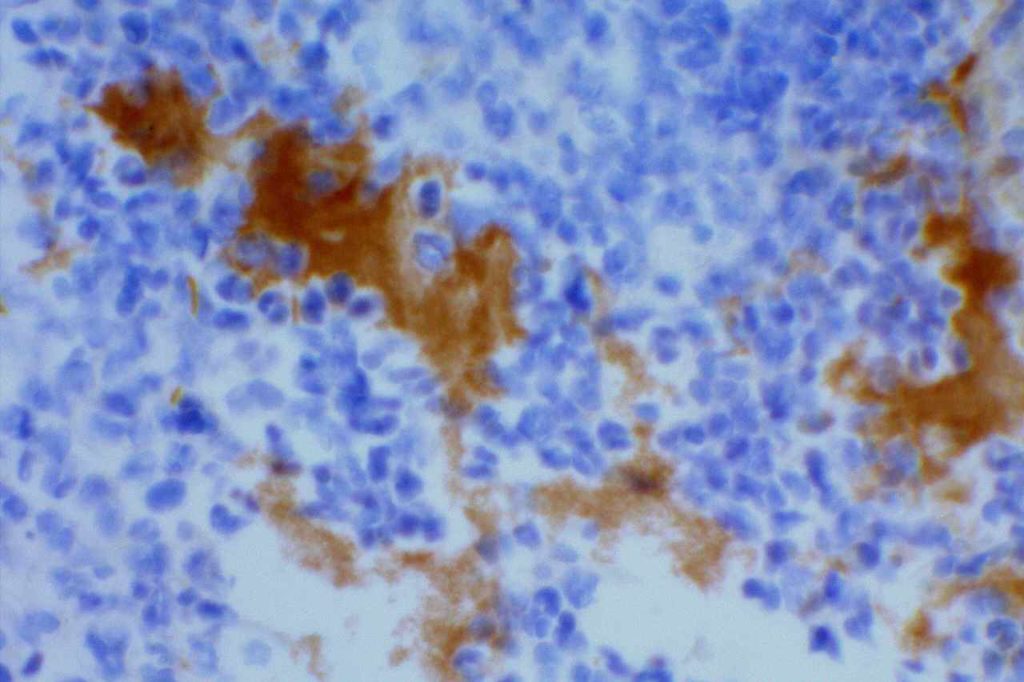
Herpes Simplex Virus (HSV)
Herpes Simplex Virus (HSV) is a virus, which causes a painful skin/mucosal blistering disorder that can involve numbers areas including genitalia, lips, esophagus, and skin. Immunohistochemistry (IHC) antibodies for HSV-1 and HSV-2 are available for testing in paraffin embedded tissue. In general, HSV-1 infections are more common above the diaphragm, and HSV-2 are more common below the diagram. While the availability of HSV-1 specific and HSV-2 specific antibodies for testing might suggest they are highly specific. In the author’s experience, there is a lot of cross reactivity between the HSV-1 and HSV-2 antibodies, and one should not consider positivity as definitively specific.
Photomicrographs