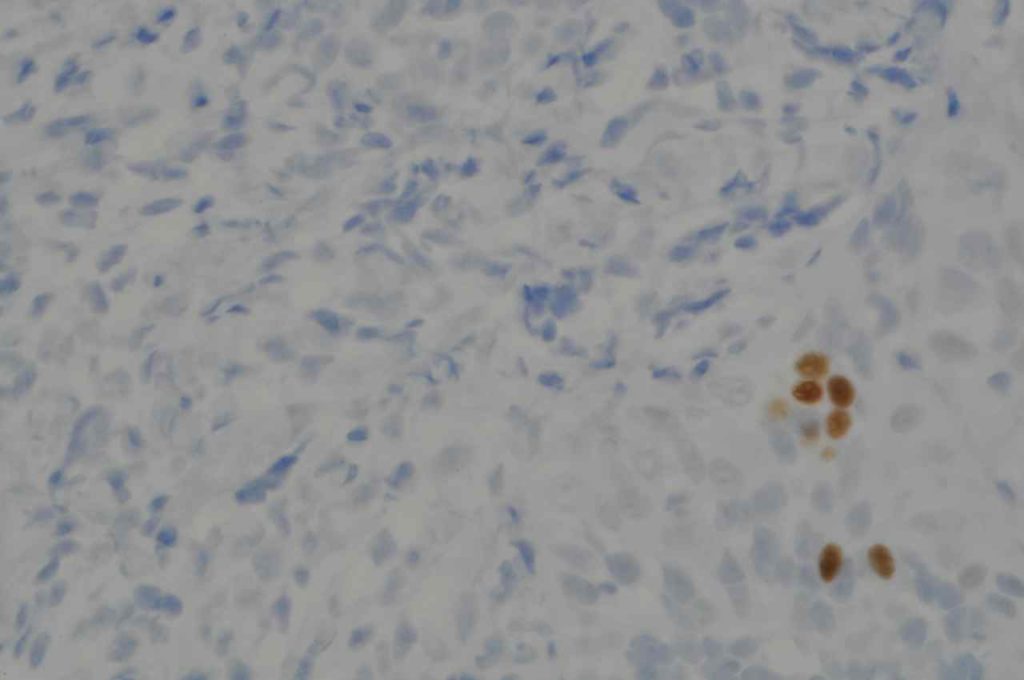
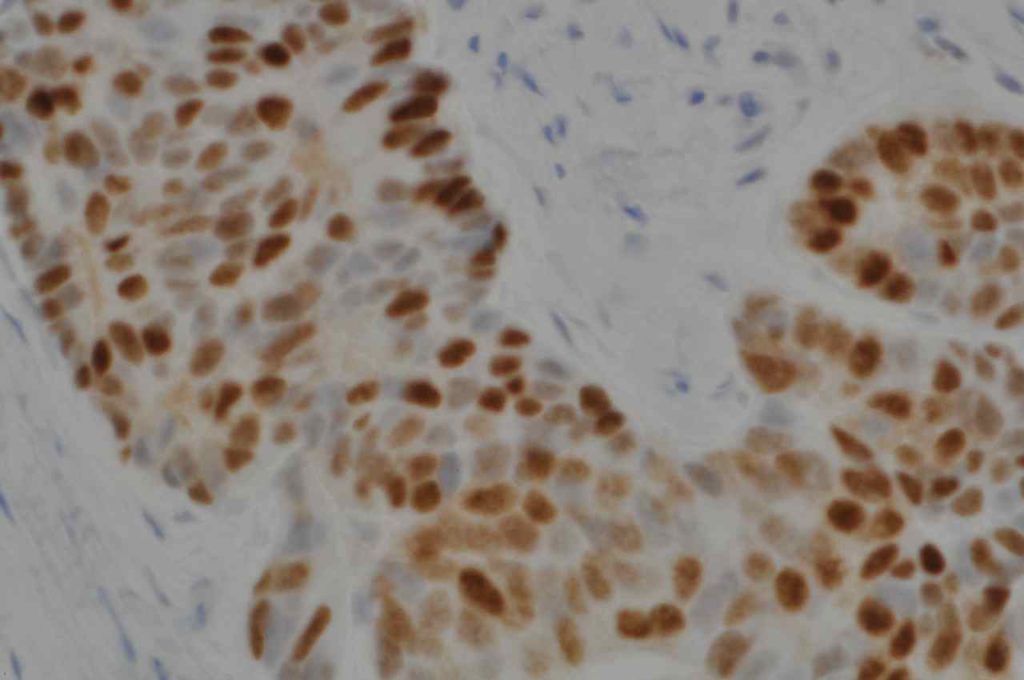
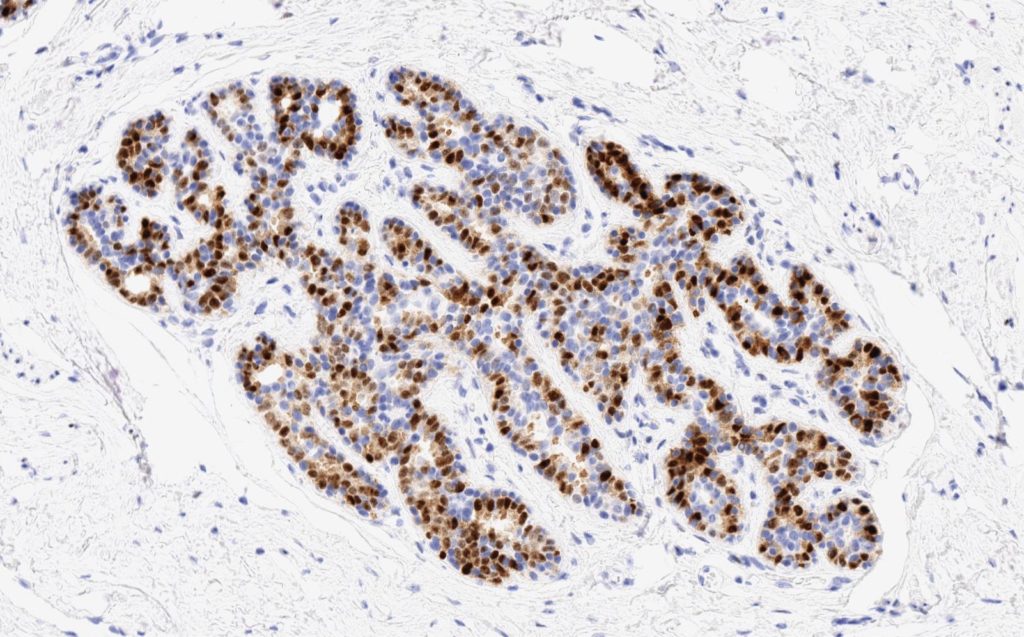
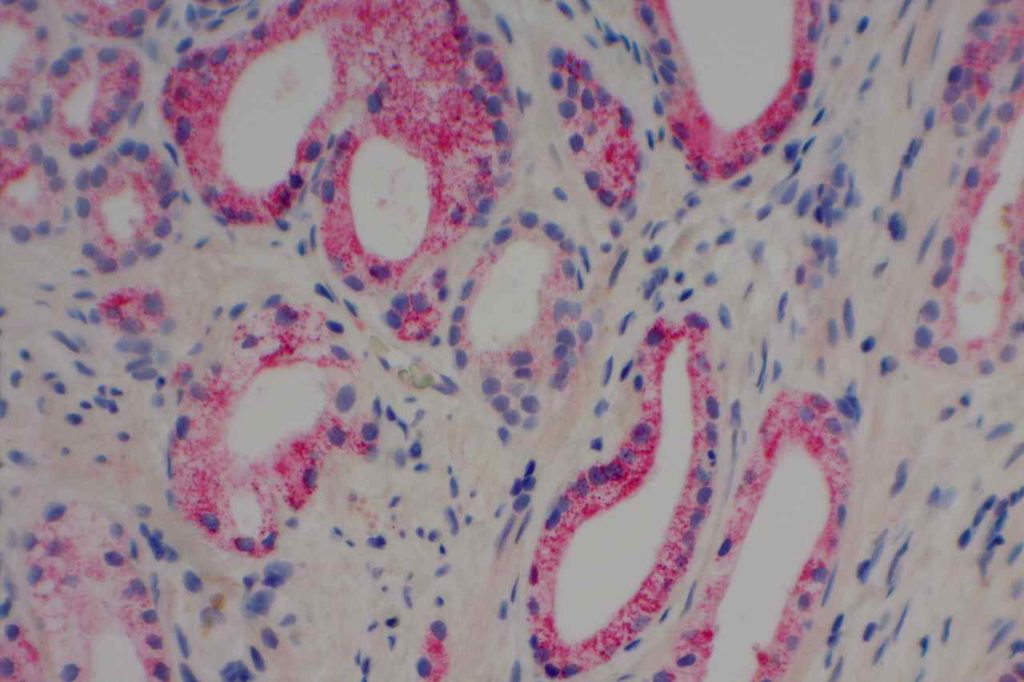
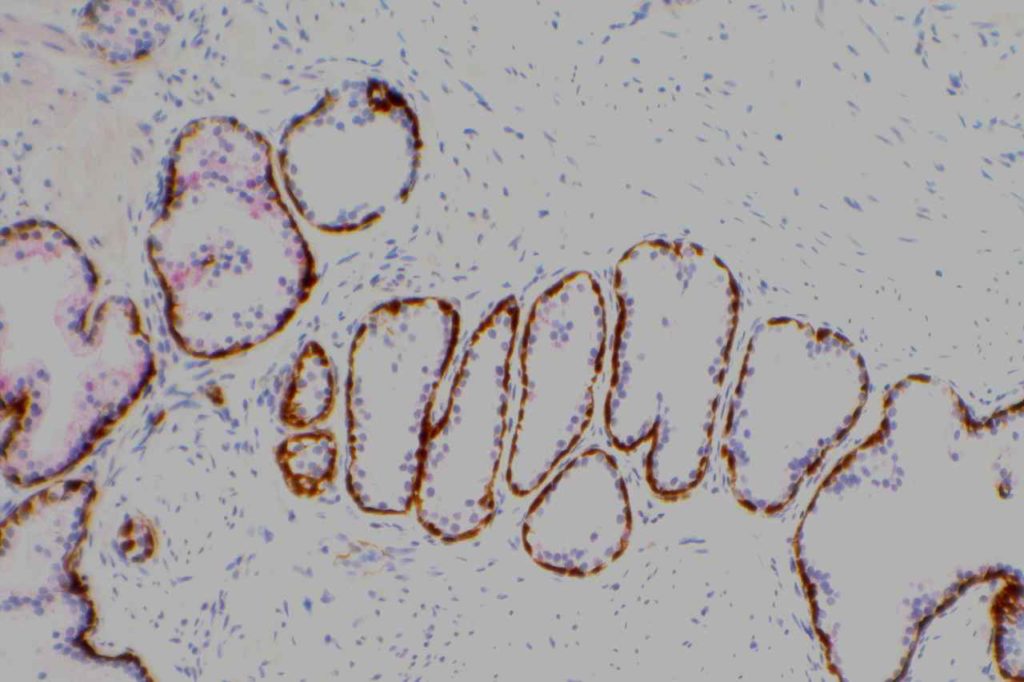
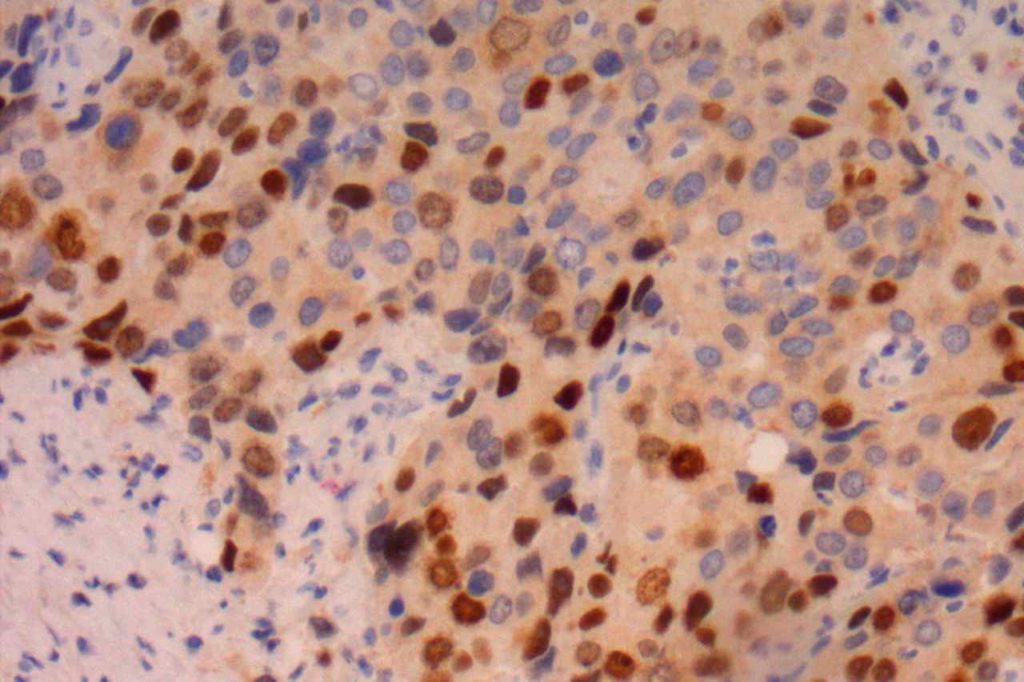
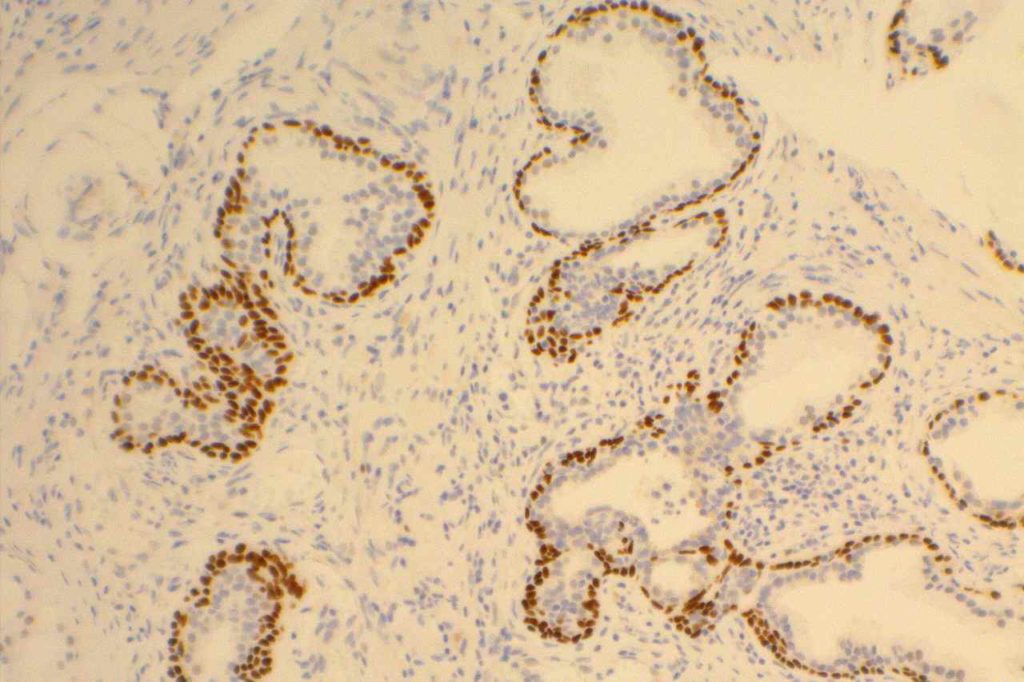
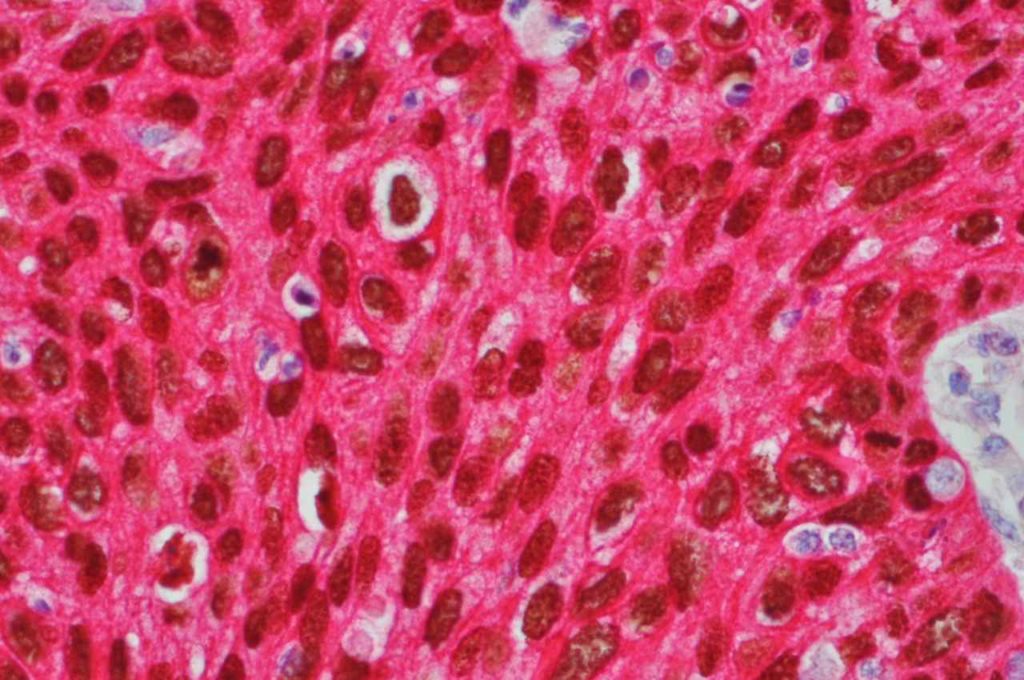
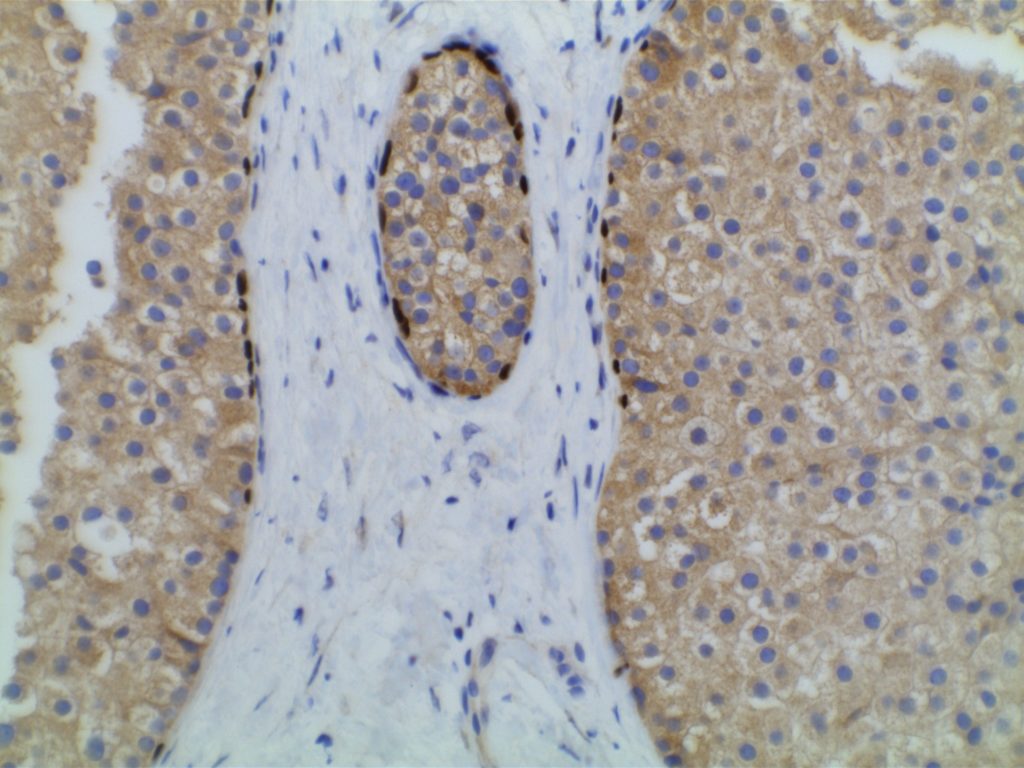
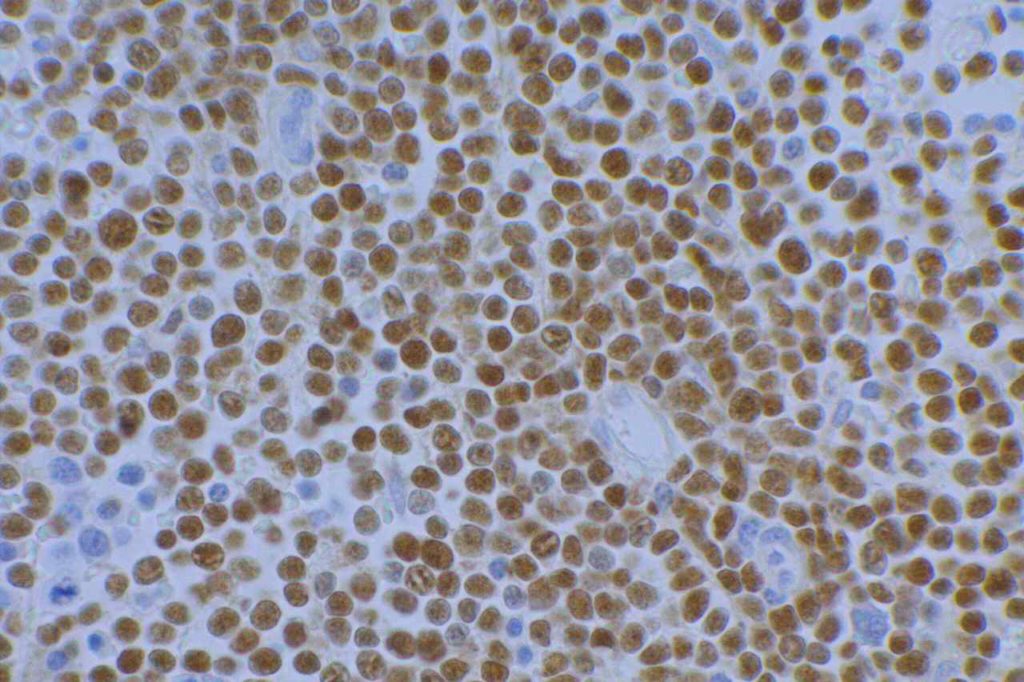
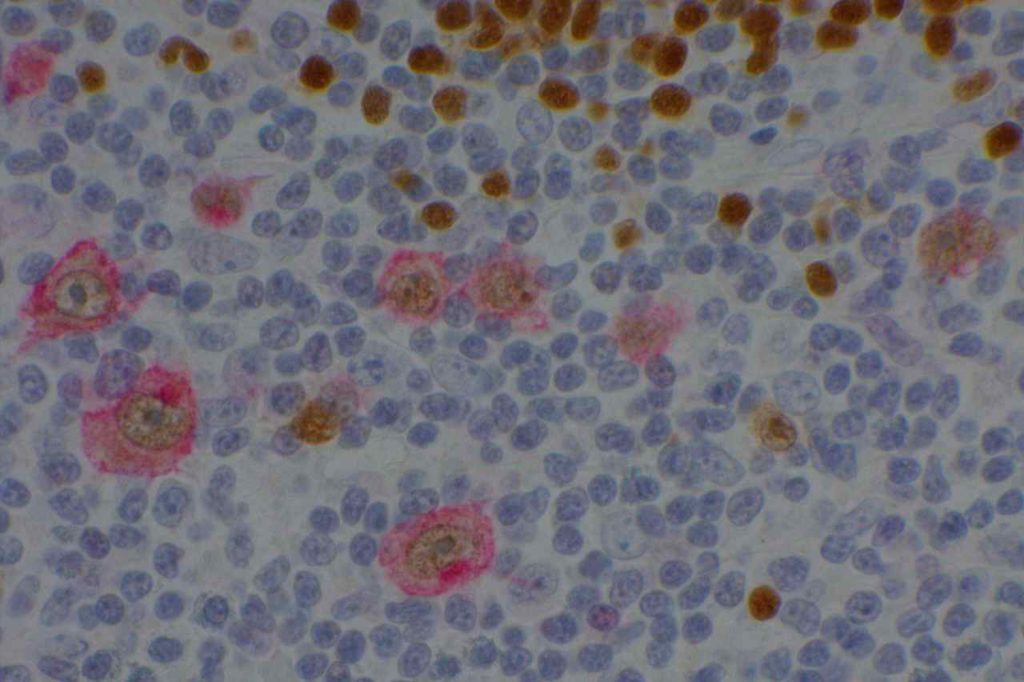
Prostate-specific membrane antigen (PSMA) is a glycoprotein, which functions as folate hydrolase, and is very specific for prostate tissue (benign and malignant). Expression can be seen with various patterns including: cytoplasmic and membraneous, cytoplasmic, apical, and apical/cytoplasmic. It appears fairly specific for prostate, although Mhawech-Fauceglia, et al. found 17% of urothelial carcinomas to have cytoplasmic expression. In non-prostate normal tissues, PSMA showed weak cytoplasmic expression in endometrial glands, testis, bladder, and kidney tubules. Moderate cytoplasmic expression was seen in pancreas islets, GI tract ganglion cells, and brain, and strong cytoplasmic expression was seen in heart tissue.
Mhawech-Fauceglia, et al. only showed 66% sensitivity for PSMA, but this study was performed on 6 mm core punch tissue-microarray (TMA) using the YPSMA-1 clone (1:50, GeneTex, Inc., San Antonio, TX). Mohanty, et. al. showed 100% sensitivity for PSMA in a study of poorly differentiated prostate andenocarcinomas of the bladder neck using the 1D6 clone (1:20, Novocastra, Leica, Buffalo Grove, IL). It is unclear if the difference between the studies is the clone used, or that Mhawpch-Fauceglia, et al. used a small TMA, while Mohanty, et. al. used whole sections. Additional study is required in this area, and it is advisable if one chooses to use this antibody (or bring it into their lab), one should verify the performance in-house.
The specificity of PSMA is a bit difficult to completely characterize. Mhawpch-Fauceglia, et al. studied 987 benign tissues and 2,174 malignant tumors. A small subset (usually 15% or less) of a wide variety of tumors showed some expression (usually weak). Please refer to the references for a complete description beyond this brief summary. Expanded data on the 1D6 clone, similar to the Mhawpch-Fauceglia et al. paper on the YPSMA-1 clone, is not available to our knowledge.
|
Study
|
Prostate
Adenocarcinoma
|
Urothelial
Carcinoma
|
|
Mhawech-Fauceglia, et. al.
(YPSMA-1, 1:50, GeneTex)
|
66% + (N=141)
|
17% + (N=346)
|
|
Mohanty, et. al.
(1D6, 1:20, Novocastra)
|
100% + (N=20)
|
0% + (N=16)
|
References:
Mohanty SK, Smith SC, Chang E, et al. Evaluation of contemporary prostate and urothelial lineage biomarkers in a consecutive cohort of poorly differentiated bladder neck carcinomas. Am J Clin Pathol. 2014;142(2):173–183. doi:10.1309/AJCPK1OV6IMNPFGL.
Mhawech-Fauceglia P, Zhang S, Terracciano L, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50(4):472–483. doi:10.1111/j.1365-2559.2007.02635.x.