Category Archives: Antibody List
ROS-1 rearrangements with at least 12 different partner proteins have been identified in a small subset of lung non-small cell carcinomas (1–2%), which shows susceptibility to tyrosine kinase inhibitors (TKIs) similar to ALK rearranged tumors. ROS-1 is considered a oncogene found on chromosome 6. The exact mechanism of activation of this gene protein product with the various gene rearrangement partners is not understood. The protein function is similar to that of the ALK family, which is why this mutation was studied for possible response to ALK inhibitors (crizotinib).
Recently, ROS-1 mutated tumors have been approved for TKI therapy with identification of a rearrangement by FISH analysis. Like ALK, ROS-1 FISH utilizes a break apart probe to identify the presence of a gene rearrangement. Other successful modalities for identification of ROS-1 rearrangements include ‘next generation’ sequencing (NGS) and immunohistochemistry.
Immunohistochemistry (IHC) has been studied as an alternative to FISH as a screening modality. Based on multiple studies, the sensitivity of IHC appears to be near 100% with the specificity of at least 92%. These studies were performed using the D4D6 rabbit monoclonal antibody clone (Cell Signaling Technology, Danvers, Massachusetts).
Unlike ALK, there is no known normal tissue counterpart which can be used as a control. Therefore, known ROS-1 positive tumors or cell lines (HCC78 cell line with the SLC34A2-ROS1 rearrangement) are generally used. ROS-1 expression is cytoplasmic with described expression ranging from finely granular to globular cytoplasmic staining and membranous staining. No consensuses has been established as to the minimal threshold of positivity.
Possible interpretation pitfalls include weak staining of type II pneumocytes and alveolar macrophages along with osteoclast-type giant cells in bone biopsies. Like any immunostain, contextual evaluation is critical.
Thunnissen E, Allen TC, Adam J, Aisner DL, Beasley MB, Borczuk AC, et al. Immunohistochemistry of Pulmonary Biomarkers: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2018;142: 408–419. doi:10.5858/arpa.2017-0106-SA
Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371: 1963–1971. doi:10.1056/NEJMoa1406766
Bubendorf L, Büttner R, Al-Dayel F, Dietel M, Elmberger G, Kerr K, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016;469: 489–503. doi:10.1007/s00428-016-2000-3
Boyle TA, Masago K, Ellison KE, Yatabe Y, Hirsch FR (2015) ROS1 immunohistochemistry among major genotypes of non- small-cell lung cancer. Clin Lung Cancer 16(2):106–111. doi:10.1016/j.cllc.2014.10.003
CaoB,WeiP,LiuZ,BiR,LuY,ZhangL,ZhangJ,YangY,Shen C, Du X, Zhou X (2016) Detection of lung adenocarcinoma with ROS1 rearrangement by IHC, FISH, and RT-PCR and analysis of its clinicopathologic features. Onco Targets Ther 9:131–138. doi:10.2147/OTT.S94997
Sholl LM, Sun H, Butaney M, Zhang C, Lee C, Janne PA, Rodig SJ (2013) ROS1 immunohistochemistry for detection of ROS1-rearranged lung adenocarcinomas. Am J Surg Pathol 37(9):1441–1449. doi:10.1097/PAS.0b013e3182960fa7
Yoshida A, Tsuta K, Wakai S, Arai Y, Asamura H, Shibata T, Furuta, K, Kohno T, Kushima R (2014) Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung can- cers. Mod Pathol 27(5):711–720. doi:10.1038/modpathol.2013.192
Rogers TM, Russell PA, Wright G, Wainer Z, Pang JM, Henricksen LA, Singh S, Stanislaw S, Grille J, Roberts E, Solomon B, Fox SB (2015) Comparison of methods in the detection of ALK and ROS1 rearrangements in lung cancer. J Thorac Oncol 10(4):611–618. doi:10.1097/JTO.0000000000000465
Rimkunas VM, Crosby KE, Li D, Hu Y, Kelly ME, Gu TL, Mack JS, Silver MR, Zhou X, Haack H (2012) Analysis of receptor tyro- sine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res 18(16): 4449–4457. doi:10.1158/1078-0432.CCR-11-3351
Mescam-Mancini L, Lantuejoul S, Moro-Sibilot D, Rouquette I, Souquet PJ, Audigier-Valette C, Sabourin JC, Decroisette C, Sakhri L, Brambilla E, McLeer-Florin A (2014) On the relevance of a testing algorithm for the detection of ROS1-rearranged lung adenocarcinomas. Lung Cancer 83(2):168–173. doi:10.1016/j. lungcan.2013.11.019
Shan L, Lian F, Guo L, Qiu T, Ling Y, Ying J, Lin D (2015) Detection of ROS1 gene rearrangement in lung adenocarcinoma: comparison of IHC, FISH and real-time RT-PCR. PLoS One 10(3): e0120422. doi:10.1371/journal.pone.0120422
OCT-2
Octamer-binding transcription factor 2 (OCT-2) is a B-cell transcription factor (nuclear marker), which interacts with BOB.1 as a cotranscription factor for the activation of the immunoglobulin promoter in the process of immunoglobulin gene expression.
Calcitonin
Calcitonin is a neuroendocrine marker expressed in medullary carcinoma of the thyroid, and thus makes up the great majority of use in diagnostic immunohistochemistry. Continue reading Calcitonin
MOC-31
MOC-31 is a glycoprotein on the cell-membrane (epithelial glycoprotein 2/epithelial cell adhesion molecule – Ep-CAM) that is widely distributed on epithelial cells and tumor cells. MOC-31 is often used to differentiate adenocarcinoma (93% positive) from mesothelioma (93% negative). Other tumors also typically negative for MOC-31 include: hepatocellular carcinoma (HCC), germ cell tumors, and renal cell (some report up to 50%+) carcinomas. Some tumors may have characteristic staining patterns (e.g. membraneous vs. cytoplasmic or apical).
MUM-1/IRF-4
MUM-1/IRF-4 (multiple myeloma oncogene 1/Interferon Regulatory Factor-4) is a nuclear transcription factor, which is expressed in late stage germinal center cells (as bcl-6 expression begins to be down-regulated) and post-germinal center lymphocytes/plasma cells. Activated T-cells may also express MUM-1.
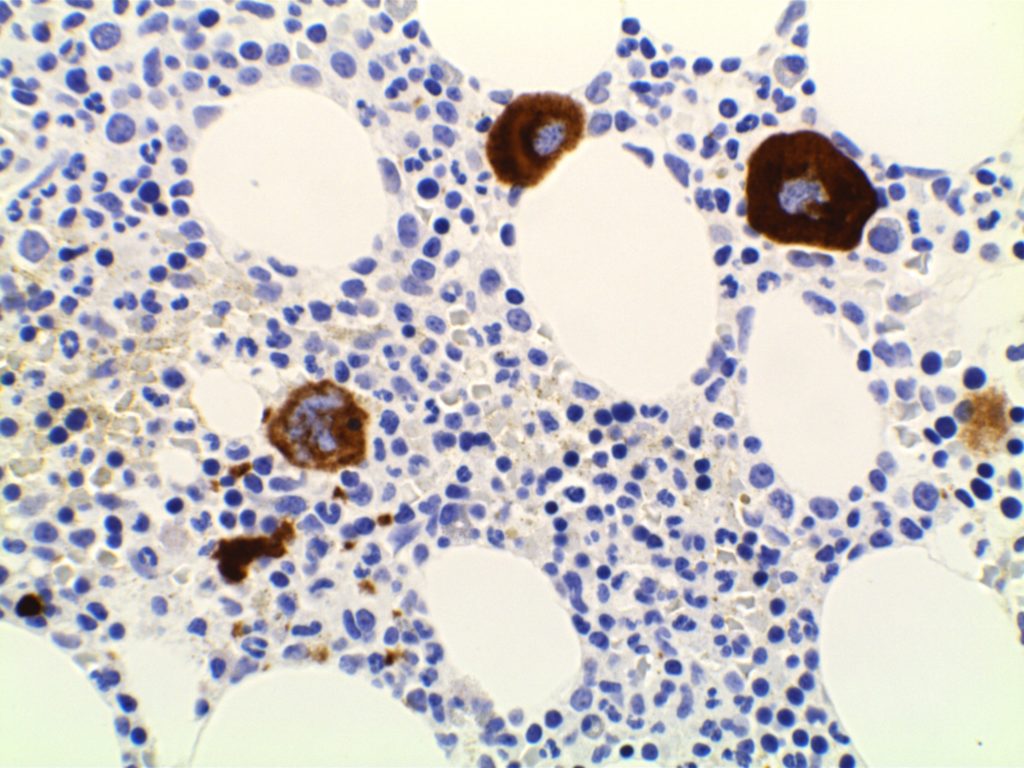
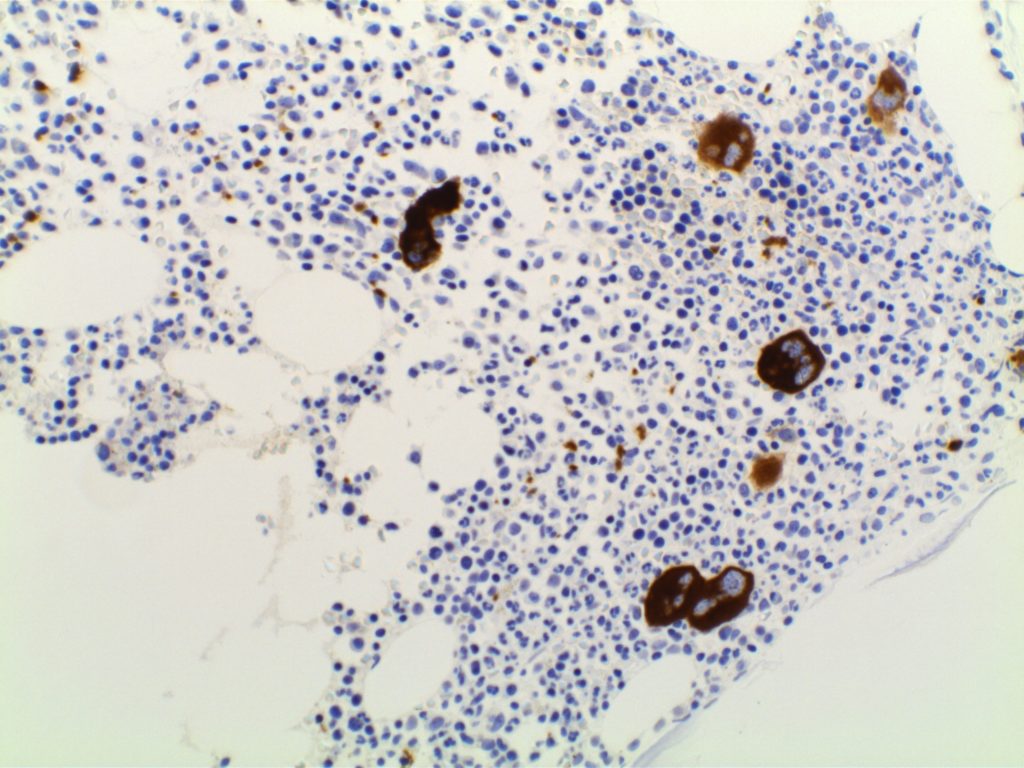
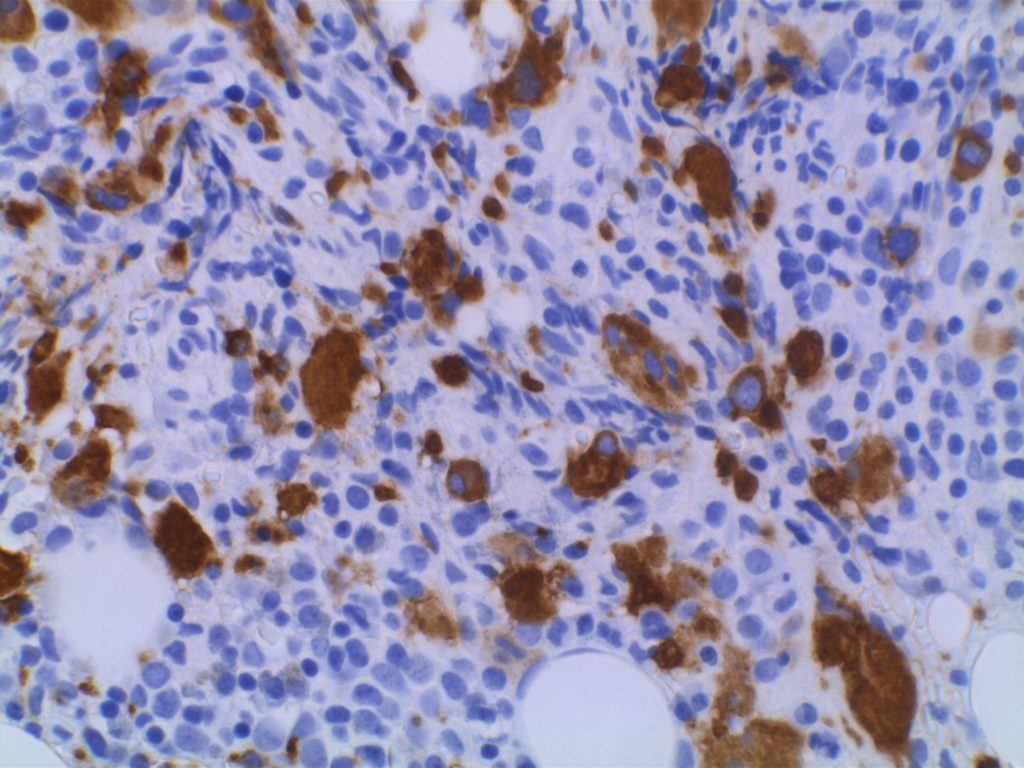
CD61
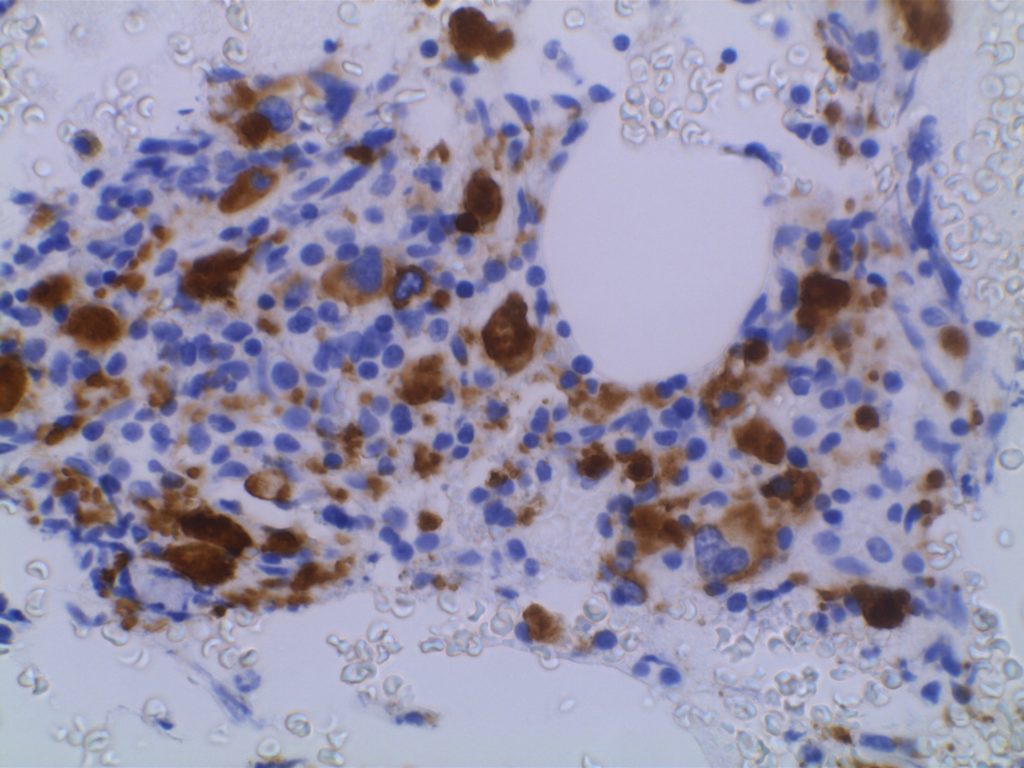
CD61 is a marker of megakaryocytes/platelets. The antigen is glycoprotein IIIa, and any cellular proliferation with megakaryocytic differentiation may express CD61. This marker has been found useful in bone marrow biopsies looking for identifying highlighting abnormal and micromegakaryocytes, which is found in some cases of myelodysplasia (MDS) and myeloproliferative neoplasms (MPN).
Some data suggests that at least 25% of the staining megakaryocytes should be micromegakaryocytes to have specificity for MDS (normal bone marrow <10% micromegakaryocytes). The opinion of some expert hematopathologists is that a significant proportion for MDS is >50% micromegakaryocytes.
Photomicrographs
References
Quitmann H, Wagner S, Fischer R. Dysmegakaryopoiesis in myelodysplastic syndromes (MDS): an immunomorphometric study of bone marrow trephine biopsy specimens. Journal of Clinical Pathology. 1991.
Chuang SS, Li CY. Useful Panel of Antibodies for the Classification of Acute Leukemia by lmmunohistochemical Methods in Bone Marrow Trephine Biopsy Specimens. Am J Clin Pathol. 1997.
Jawad MD, Go RS, Reichard KK, Shi M. Increased Multinucleated Megakaryocytes as an Isolated Finding in Bone Marrow: A Rare Finding and Its Clinical Significance. Am J Clin Pathol. 2016;146: 561–566. doi:10.1093/ajcp/aqw144
FOX, S.B., LORENZEN, J., HERYET, A., JONES, M., GATTER, K.C. and MASON, D.Y. (1990), Megakaryocytes in myelodysplasia: an immunohistochemical study on bone marrow trephines. Histopathology, 17: 69–74. doi:10.1111/j.1365-2559.1990.tb00665.x
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 109-113.
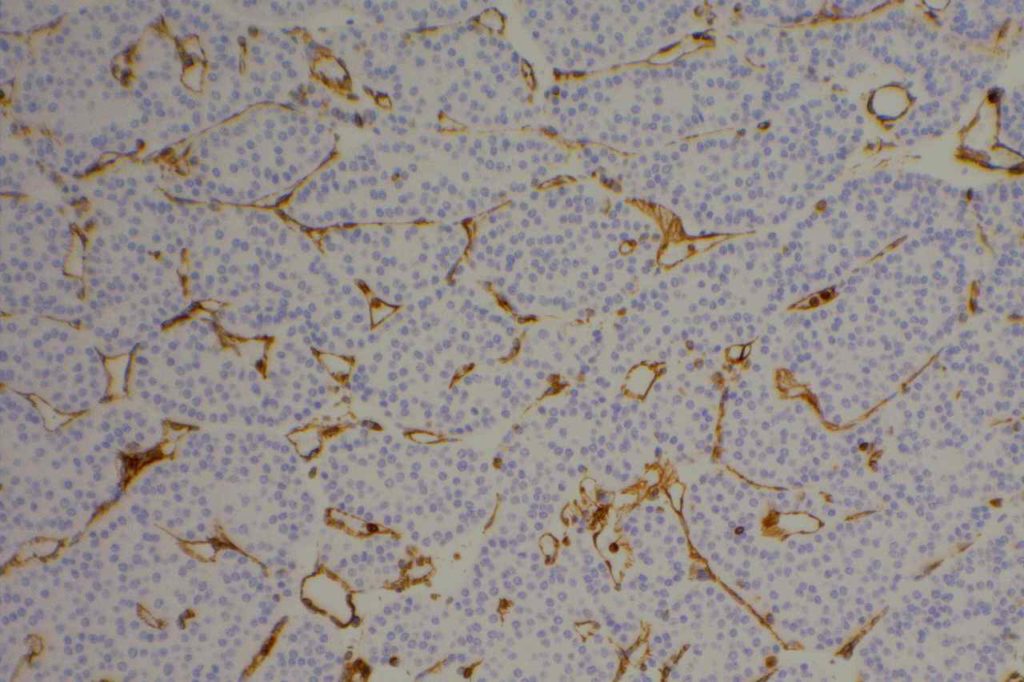
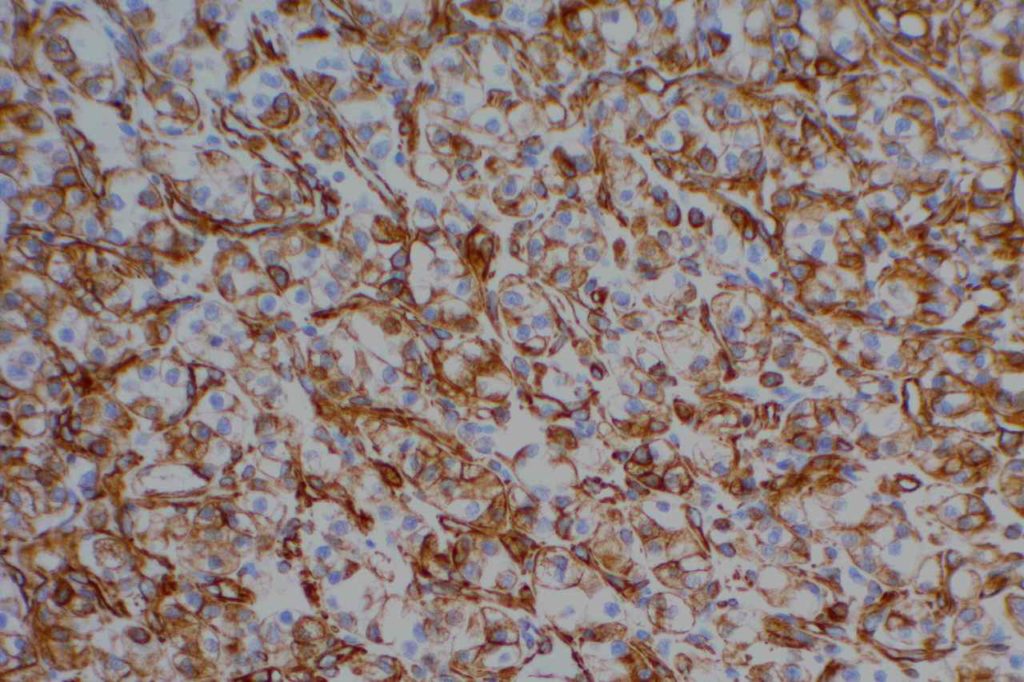
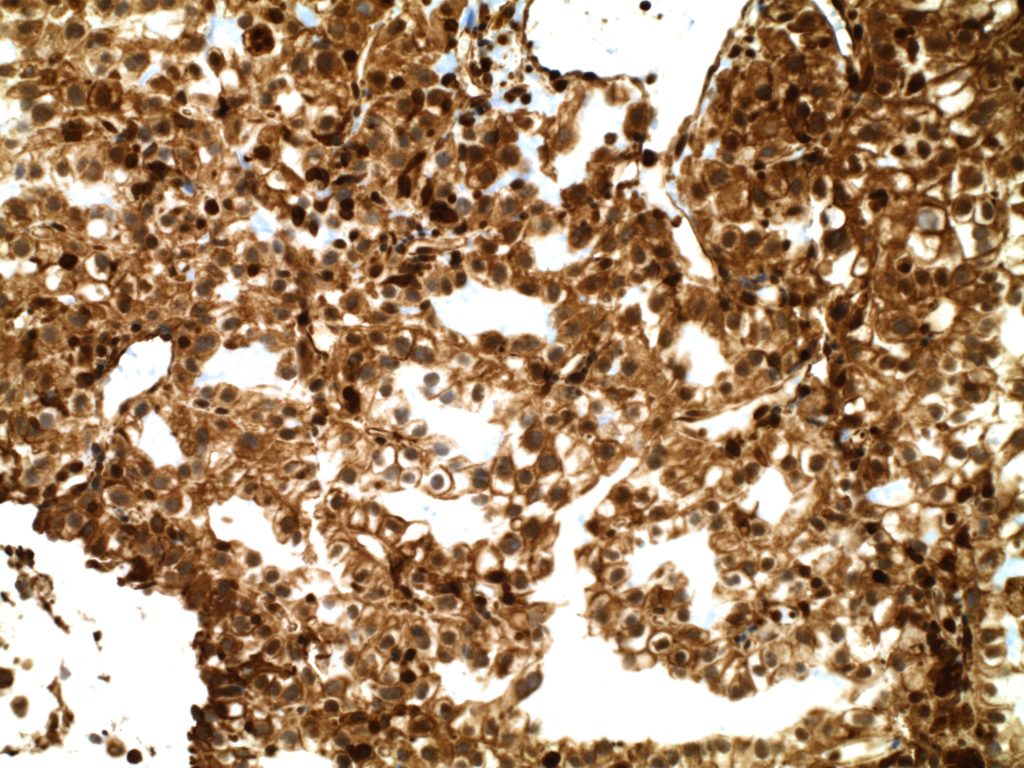
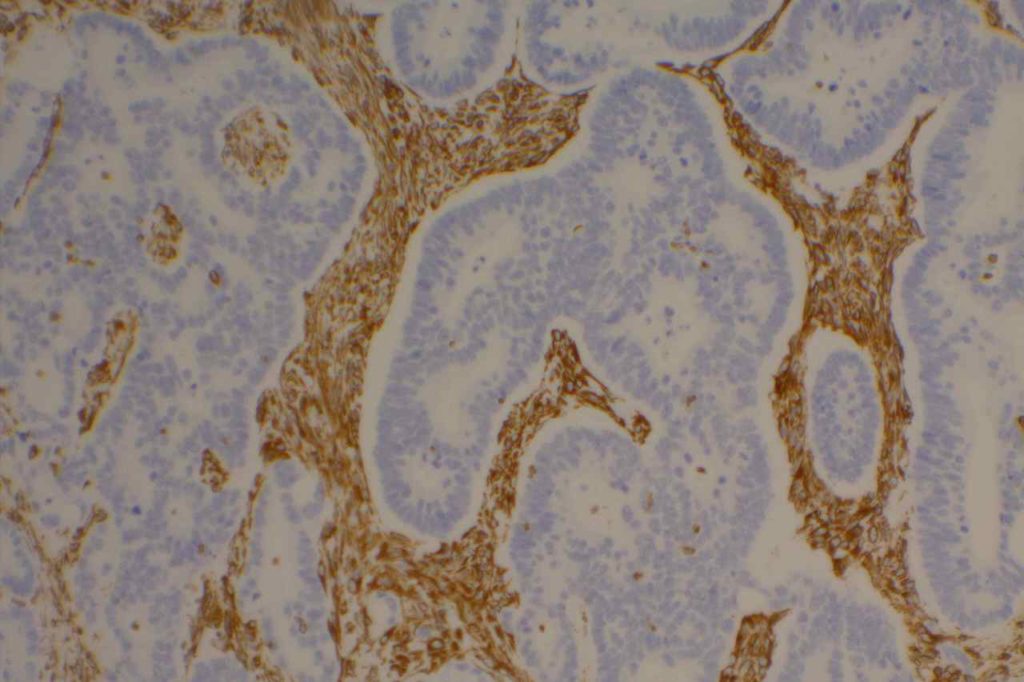
Vimentin
Vimentin is an intermediate filament found in mesenchymal tissue. It is not a specific stain and there is a subset of tumors which characteristically have co-expression of cytokeratin and vimentin. It should also be noted that poorly differentiated carcinomas of any origin may express vimentin.
Some pathologists joke they use vimentin to see if IHC stains will even work in a particular piece of tissue, but vimentin can be very helpful especially when used in conjunction with a panel of stains.
Carcinomas with co-expression of vimentin
- Renal Cell Carcinoma
- Thyroid Carcinoma (Papillary & Anaplastic)
- Endometrial Adenocarcinoma
- Sarcomatoid Carcinoma
- Mesothelioma (biphasic)
- Metaplastic Breast Carcinoma
- Myoepithelial Carcinoma
Sarcomas with co-expression of cytokeratin
- Desmoplastic Small Round Cell Tumor
- Epithelioid Sarcoma
- Epithelioid Angiosarcoma
- Malignant Rhabdoid Tumor
- Synovial Sarcoma
- Leiomyosarcoma
- Chordoma
- Adamantinoma
Photomicrographs
References
Kandukuri SR, Lin F, Gui L, Gong Y, Fan F, Chen L, et al. Application of Immunohistochemistry in Undifferentiated Neoplasms: A Practical Approach. Arch Pathol Lab Med. 2017;141: 1014–1032. doi:10.5858/arpa.2016-0518-RA
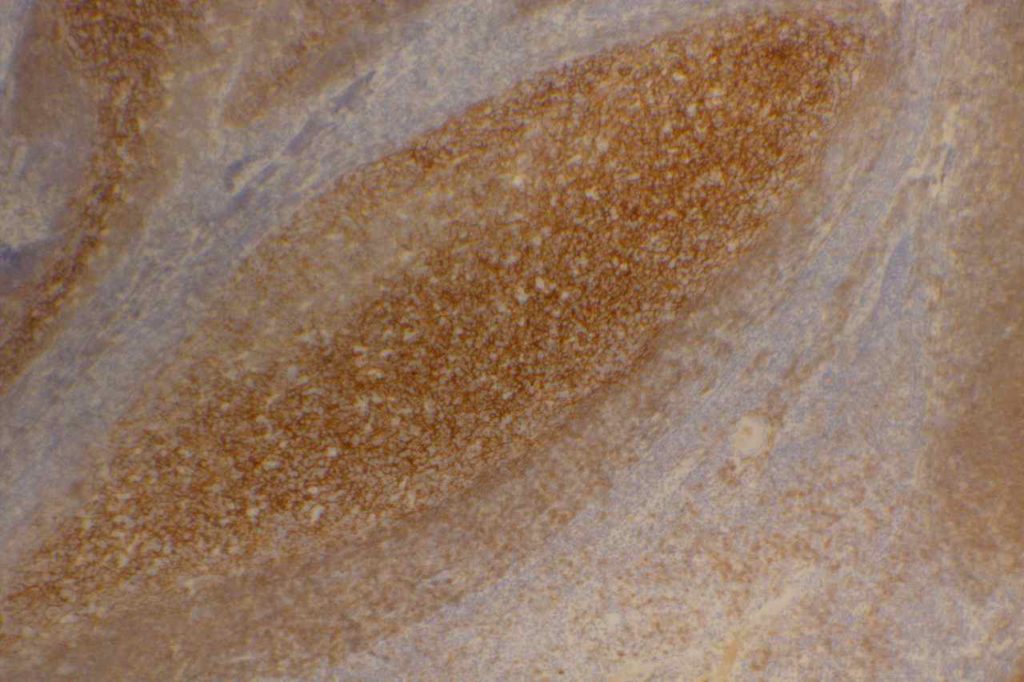
CD21
CD21 is a follicular dendritic cells (FDC) marker. It has a membranous staining pattern, but this is practically difficult to separate from a cytoplasmic pattern in lymphoid tissue. This stain is most often used as an “architectural” marker or aberrant patterns (nodular lymphocyte predominate Hodgkin lymphoma). There are some rare tumors of FDC, which will mark with CD21. CD21 may rarely mark normal B-cells, and strong expression of CD21 in CLL has been associated with a most aggressive disease course (unsure if this is by flow cytometry and/or IHC).
Follicular Lymphoma – It is also helpful to identify the expanded follicular dendritic meshwork in cases of follicular lymphoma (especially helpful in diagnostically challenging cases – i.e. identifying a follicular component in an otherwise diffuse process or in small needle biopsies where architecture may not be visible).
Angioimmunoblastic T-cell Lymphoma (AILT) – Extrafollicular dendritic cells in the paracortical region in associated with neoplastic T-cells and high endothelial venules is characteristic of AILT. CD21 is a helpful marker to highlights the follicular dendritic component in this process.
Marginal Zone Lymphoma – CD21 is useful in highlighting follicular colonization by marginal zone cells, which may be obscured by morphology alone.
CD21 Normal Expression Pattern
- Follicular Dendritic Cells
- Rare Normal B-cells
- Rare cases of CLL
- Generally thought of as a better follicular dendritic marker compared to CD23 (less sensitive)
Photomicrographs
References
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 64.
MD DY-PW, BacSc F. A case of t (14; 18)-negative follicular lymphoma with atypical immunophenotype: usefulness of immunoarchitecture of Ki67, CD79a and follicular dendritic cell meshwork in making the diagnosis. Malaysian Journal of Pathology. 2014. p. 125-129.
Harris NL, Swerdlow SH, Jaffe ES, et al. FollicularLymphoma. In: Swerdlow SH, Campo E, Harris NL,Jaffe ES, Pileri SA, Stein H, Thiele J, VardimanJW, editors. WHO classication of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press; 2008. p. 220–6.
Troxell ML, Schwartz EJ, van de Rijn M, Ross DT, Warnke RA, Higgins JP, et al. Follicular dendritic cell immunohistochemical markers in angioimmunoblastic T-cell lymphoma. Appl Immunohistochem Mol Morphol. 2005;13: 297–303.
Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21: 116–131. doi:10.1097/PAI.0b013e31825d550a
CD33
CD33 (gp67 and p67) is a specific hematopoietic marker, which is expressed in monocytes/macrophages, granulocyte precursors (decreases with maturation), mast cells, and dendritic cells.
Diagnostically, CD33 is helpful in differentiating myeloid tumors for lymphoid neoplasms. Myelomonocytic and monoblastic leukemias may mark with CD33, but are usually negative for myeloperoxidase (MPO). Hematodermic neoplasm is also negative for CD33.
References
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 84.
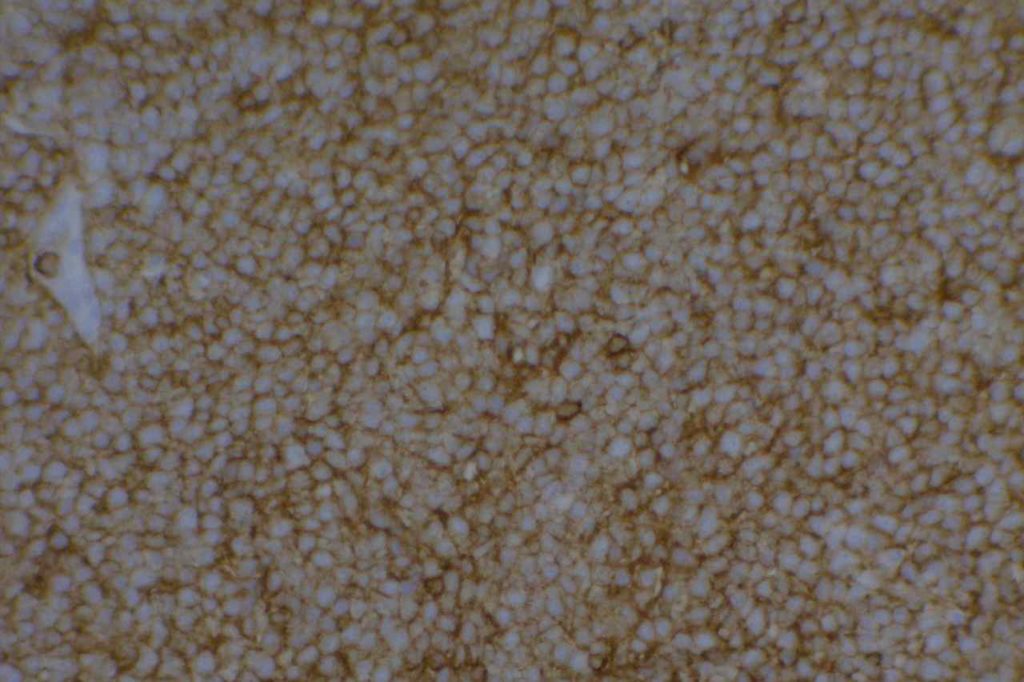
CD43
CD43 is a pan T-cell marker, which is also expressed in myeloid (granulocytic) sarcomas and some B-cell lymphomas. CD43 is aberrantly expressed in a number of B-cell lymphomas (expression is highly correlated with CD5), but this marker is most helpful in cases of marginal zone lymphoma when flow cytometry is not performed (i.e. usually small mucosal biopsies). These cases may be difficult to differentiate from lymphoid hyperplasia. Approximately 50% of cases of marginal zone lymphoma will express CD43. Negativity for CD43 does not exclude the diagnosis, and additional molecular testing may be helpful to identify these cases.
Lee et. al identified normal co-expression of CD43 (strong expression) in perifolliuclar B-cells within submucosal lymphoid tissue from terminal ileum biopsies. Therefore, CD43 co-expression on terminal ileum biopsies should NOT be used as a criterion alone to favor a diagnosis of MALT lymphoma.
The main take home point is that CD43 is a good marker, which is helpful, but there is lack a specificty and one should understand the stain performance in the diagnostic setting. In the workup of a mature B cell lymphoma, CD43 (like CD5) should be compared against CD3 and CD20 staining.
CD43 Expression in Non-Hodgkin Lymphoma (Lai R., et. al. N=742 cases)
|
Diagnosis
|
CD43 Expression (%)
|
|
>90%
|
|
|
>90%
|
|
|
>90%
|
|
|
>90%
|
|
|
20-40%
|
|
|
20-40%
|
|
|
20-40%
|
|
|
Burkitt-Like B-Cell Lymphoma
|
20-40%
|
|
20-40%
|
|
|
0-6%
|
|
|
0-6%
|
In the skin CD43 marked >95% of cases of granulocytic sarcoma (leukemia cutis). Other non-T cell lesions which may stain with CD43 include: AML, hemangioma, Langerhans cell histiocytosis, mast cell disease and plasmacytoma.
Photomicrographs
References
Lai, R., Weiss, L. M., Chang, K. L., & Arber, D. A. (1999). Frequency of CD43 expression in non-Hodgkin lymphoma. A survey of 742 cases and further characterization of rare CD43+ follicular lymphomas. American Journal of Clinical Pathology, 111(4), 488–494.
Treasure, J., Lane, A., Jones, D. B., & Wright, D. H. (1992). CD43 expression in B cell lymphoma. Journal of Clinical Pathology, 45(11), 1018–1022.
Cronin, D. M. P., George, T. I., & Sundram, U. N. (2009). An updated approach to the diagnosis of myeloid leukemia cutis. American Journal of Clinical Pathology, 132(1), 101–110. doi:10.1309/AJCP6GR8BDEXPKHR
Jung G, Eisenmann J-C, Thiébault S, Hénon P. Cell surface CD43 determination improves diagnostic precision in late B-cell diseases. Br J Haematol. 2003;120: 496–499.
Lee P-S, Beneck D, Weisberger J, Gorczyca W. Coexpression of CD43 by benign B cells in the terminal ileum. Appl Immunohistochem Mol Morphol. 2005;13: 138–141.
Diagnostic Immunohistochemistry: Theranostic and Genomic Applications [edited by] David J. Dabbs. 3rd Edition. pp. 165.