CD20 is a pan B-cell marker and is one of the most utilized hematopathology stains. The staining pattern morphology is membraneous and cytoplasmic. The sensitivity and specificity for mature B-cell neoplasms is very high.
Category Archives: Antibody List
CD19
CD19 is a B-cell marker. Until recently this antibody has only been available for use in flow cytometry. Now CD19 is available as an immunohistochemistry (IHC) marker, with similar sensitivity for B-cells to CD20. The IHC expression pattern is cytoplasmic and membranous. It is normally expressed on both Precursor B-cells and B-cells. CD19 combined with TdT would be specific to detect precursor B-cells. CD20 is not expressed on precursor B-cells, but mature B-cell with surface light chain expression (usually).
CD15 (Leu-M1)
CD15 (Leu-M1) is a hematopoietic antigen expressed by granulocytes, monocytes, Reed-Sternberg cells, and subsets of T and B-cells. CD15 may also be expressed in glandular or neuroendocrine epithelial cells as well as part of a panel to distinguish between metastatic adenocarcinoma (positive) and mesothelioma (negative).
CD15 Expression
- Neutrophils (~90%)
- Monocytes (30-60%)
- Promyelocytes
- Adenocarcinoma (72%)
- Classical Hodgkin Lymphoma cells
- T-cell Lymphomas (subset, MF)
- Acute Myelogenous Leukemia (particularly with monocytic differentiation)
Classical Hodgkin Lymphoma
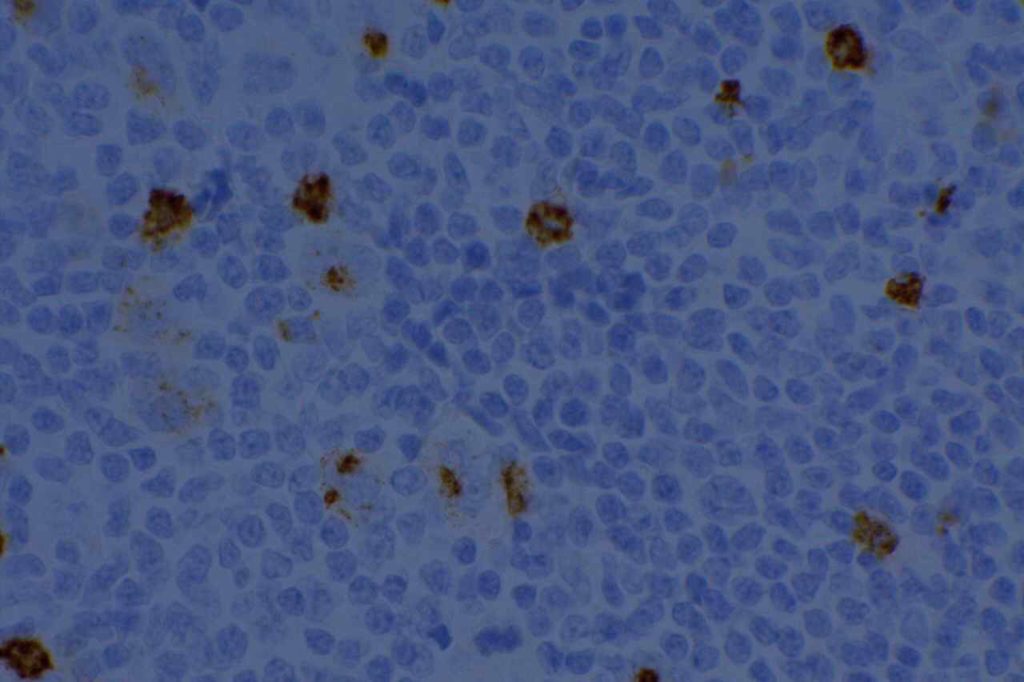
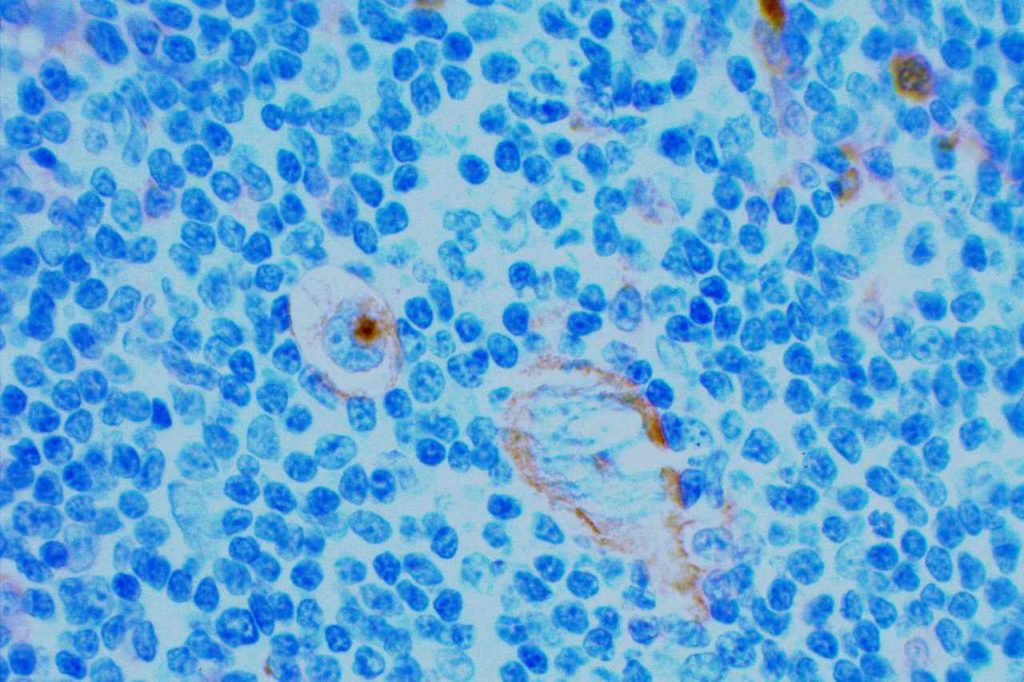
CD15 stains strongly the cytoplasm of granulocytes. CD15 is most commonly used as a diagnostic marker for classical Hodgkin lymphoma (CHL). The combined staining of CD15 and CD30 in the appropriate morphologic setting is very specific for Hodgkin Lymphoma. The biggest issue with this marker in the diagnosis of CHL is sensitivity. In a well-optimized setting using a preferred clone, the sensitivity can be fairly high, ~70%. In general when CHL cases are positive for CD15, it will stain a subset of the CD30 positive cells.
When this marker is not well optimized and/or using a suboptimal clone, then the negative results can not be trusted (even when expressed in background granulocytes). Comparison analysis with a reference lab to compare IHC expression in validation cases is recommended for this antibody. PAX-5 is also a helpful marker, when CD15 is negative and CHL is suspected, as it will be dimly expressed (compared to background B-cells) in ~90% of CHL cases.
Bone Marrow
CD15 will stain at least a subset of normal granulocytes(~90%), monocytes (30-60%), promyelocytes in the bone marrow. A subset of cases of acute myelogenous leukemia (AML) will express CD15. CD15 is a myeloid marker, and appears to be preferentially expressed in AML cases with monocytic differentiation (not entirely sensitive or specific). Flow cytometry is more sensitive in detected CD15 expression than immunohistochemistry (IHC). A subset of neoplastic T and B-cells may also express CD15.
Mesothelioma vs. Adenocarcinoma
CD15 has been used as part of a panel to differentiate between mesothelioma and adenocarcinoma. CD15 is expressed in ~7% of epithelioid mesotheliomas and is not known to be expressed in sarcomatous mesothelioma while being expressed in a majority of adenocarcinomas (~72%). CD15 is not typically one of the main markers used in the mesothelioma vs. adenocarcinoma panel (there are other markers with better sensitivity/specificity profiles), but may be helpful in certain circumstances.
CD15 Expression (Marchevsky)
- Epithelial mesothelioma – 7%
- Sarcomatous mesothelioma – 0%
- Adenocarcinoma – 72%
- Squamous cell carcinoma – 30%
- Renal cell carcinoma – 25-100%
Photomicrographs
References
Wick, MR. “Immunohistochemical approaches to the diagnosis of undifferentiated malignant tumor.”Annals of Diagnostic Pathology12(2008):72-84.
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 51.
Marchevsky, A. M. (2008). Application of immunohistochemistry to the diagnosis of malignant mesothelioma. Archives of Pathology & Laboratory Medicine, 132(3), 397–401.
Dunphy CH, Polski JM, Evans HL, Gardner LJ. Evaluation of bone marrow specimens with acute myelogenous leukemia for CD34, CD15, CD117, and myeloperoxidase. Arch Pathol Lab Med. 2001;125: 1063–1069.
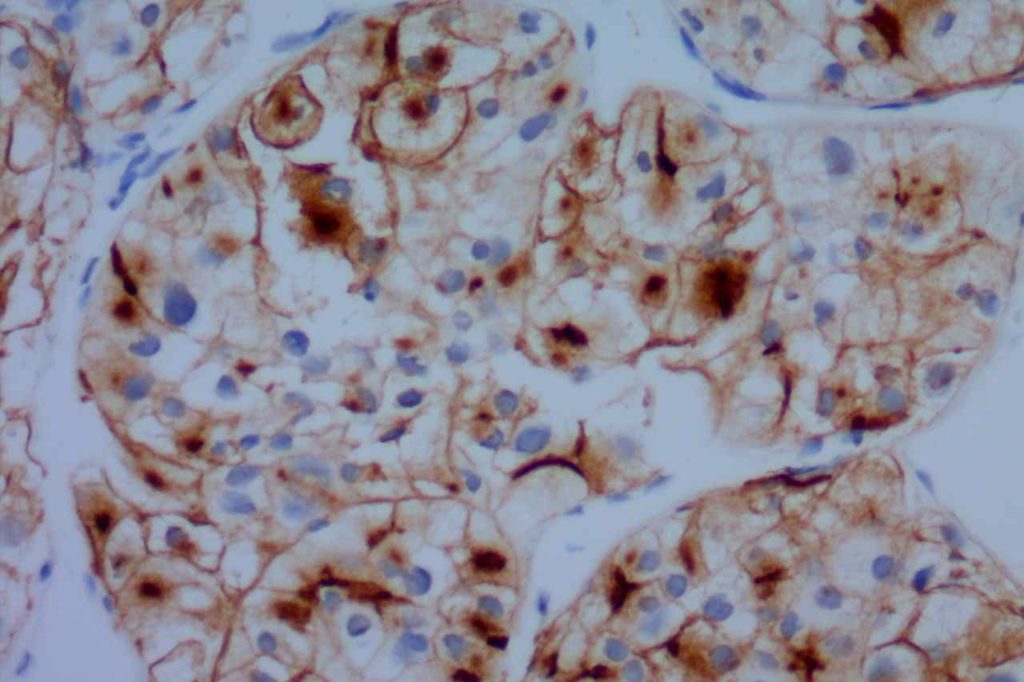
CD10
Hematopathology
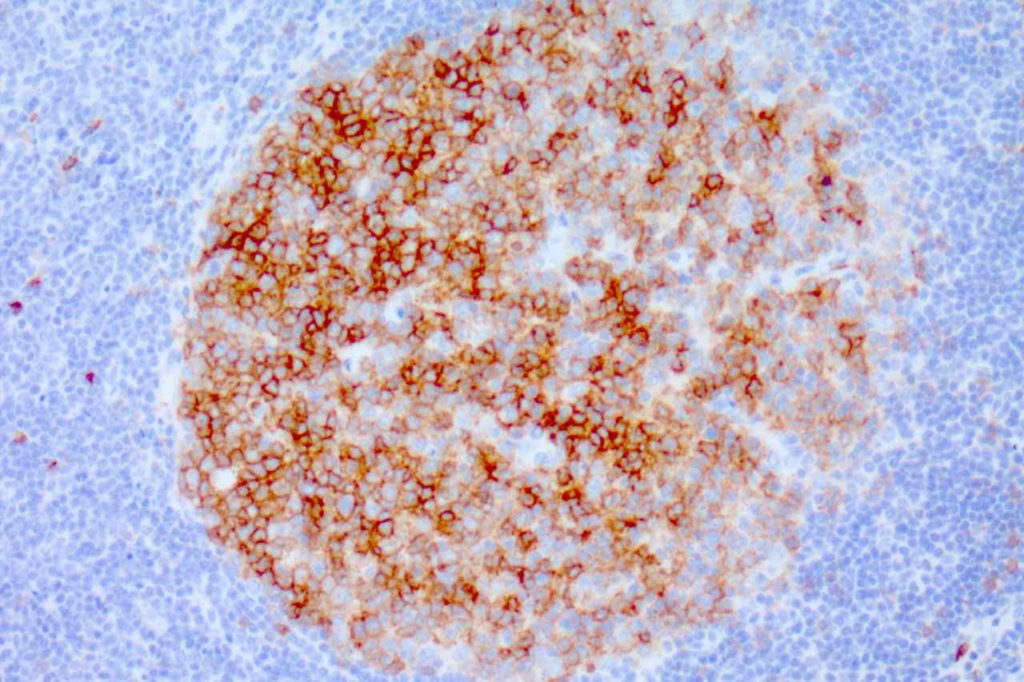
CD10 (a.k.a. CALLA, common acute lymphoblastic leukemia antigen) is a useful marker for cells of germinal center cell origin and is expressed during the lymphoblastic phase of development. Therefore, this marker is diagnostically helpful in several areas in hematopathology: acute lymphoblastic leukemia (ALL), follicular lymphoma, diffuse large B-cell lymphoma (DLBCL), and Burkitt lymphoma.
ALL will often show expression of CD10. In fact, CD10 co-expression with TdT is characteristic of ALL (additional expression of T- or B-cell markers will help further classify).
CD10 is a marker of follicle center cell origin, which is characteristic of certain lymphomas including: follicular lymphoma, Burkitt lymphoma, and a subset of DLBCLs.
CD10 can be used as part of a prognostic panel (CD10, bcl-6, and MUM-1) in DLBCL to help separate cases into germinal center and non-germinal center subtypes. The Hans’ (classifier) algorithm method is the most popular, probably due to the simplicity of the algorithm and utilization of IHC markers already present in most laboratories.
Non-Hematopathology
CD10 is a useful marker in non-lymphoid malignancies: renal cell carcinoma and hepatocellular carcinoma. CD10 will have a “bile canaliculi” pattern in HCC. CD10 will also stain endometrial stromal sarcoma, and the “brush boarder” in GI tumors.
Pitfalls
CD10 can appear to have a lot of “non-specific” staining because of staining of dendritic stomal cells. This can cause a pattern similar to reticular fibers, and many describe this as a “reticular pattern,” but the staining does not directly correlate with reticulin staining. Caution should be exercised in using this stain in isolation given its lack of specificity (see below).
CD10 Expression in tumors often studied by CD10 IHC staining
- Acute Lymphoblastic Lymphoma (ALL)
- Normal Lymphoid Precursors (hematogones)
- Bone Marrow: Mature neutrophils (~25%)
- Dendritic Stromal Cells
- Follicular Lymphoma
- Diffuse Large B-cell Lymphoma (DLBCL), 30-60%
- Angioimmunoblastic T-cell Lymphoma
- Burkitt Lymphoma
- Renal Cell Carcinoma (clear cell, papillary and Xp11.2 translocation tumors)
- Endometrial stomal sarcoma
Other tumors/tissues with CD10 expression (20-100% expression)
- Hepatocellular Carcinoma
- Breast myoepithelial cells and stromal fibroblasts
- Cutaneous adnexal neoplasms
- Mesothelioma
- Epithelioid hemangioendotheliomas
- Ovarian carcinoma
- Urothelial carcinoma
- Prostatic adenocarcinoma
- Colon adenocarcinoma
- Melanoma
- Spindle cell carcinoma
- Lung carcinomas
- Pancreatic solid pseudo papillary carcinoma
Photomicrographs
References
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103: 275–282. doi:10.1182/blood-2003-05-1545
Tan, P.-H., Cheng, L., Rioux-Leclercq, N., Merino, M. J., Netto, G., Reuter, V. E., et al. (2013). Renal tumors: diagnostic and prognostic biomarkers. (Vol. 37, pp. 1518–1531). Presented at the The American journal of surgical pathology. doi:10.1097/PAS.0b013e318299f12e
Chang, C.-C., McClintock, S., Cleveland, R. P., Trzpuc, T., Vesole, D. H., Logan, B., et al. (2004). Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. The American Journal of Surgical Pathology, 28(4), 464–470.
Tan P-H, Cheng L, Rioux-Leclercq N, Merino MJ, Netto G, Reuter VE, et al. Renal tumors: diagnostic and prognostic biomarkers. 2013. pp. 1518–1531. doi:10.1097/PAS.0b013e318299f12e
Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2010;135: 92–109. Available: http://www.archivesofpathology.org/doi/pdf/10.1043/2010-0478-RAR.1
Dewar R, Fadare O, Gilmore H, Gown AM. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135: 422–429. doi:10.1043/2010-0336-CP.1
Alizadeh AA, Elsen MB, Davis RE, Ma C. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127: 2375–2390. doi:10.1182/blood-2016-01-643569
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 38.
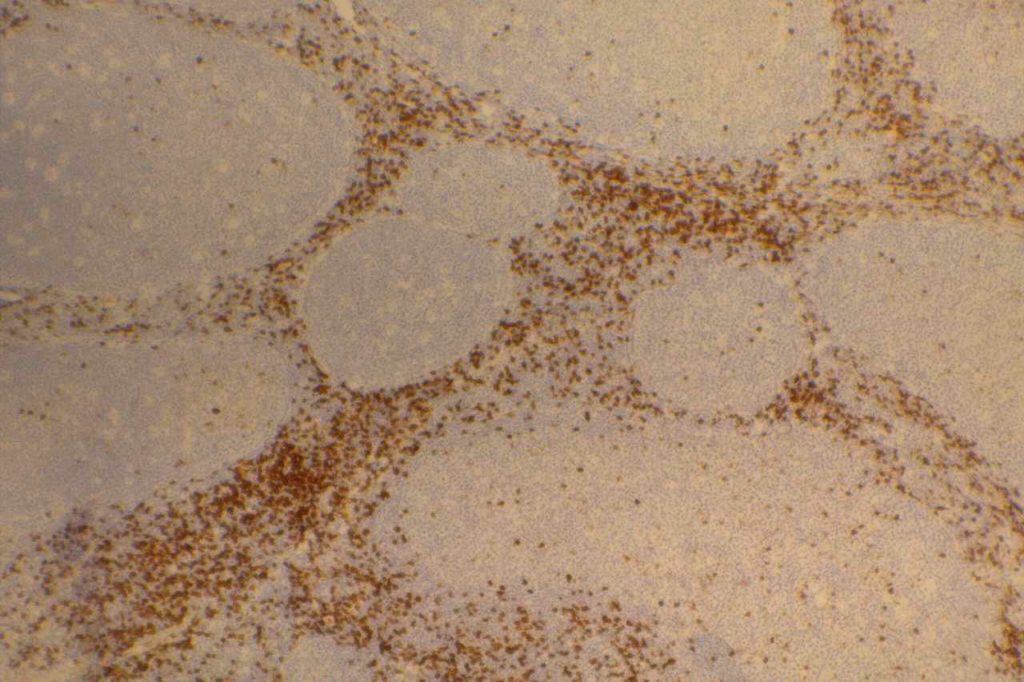
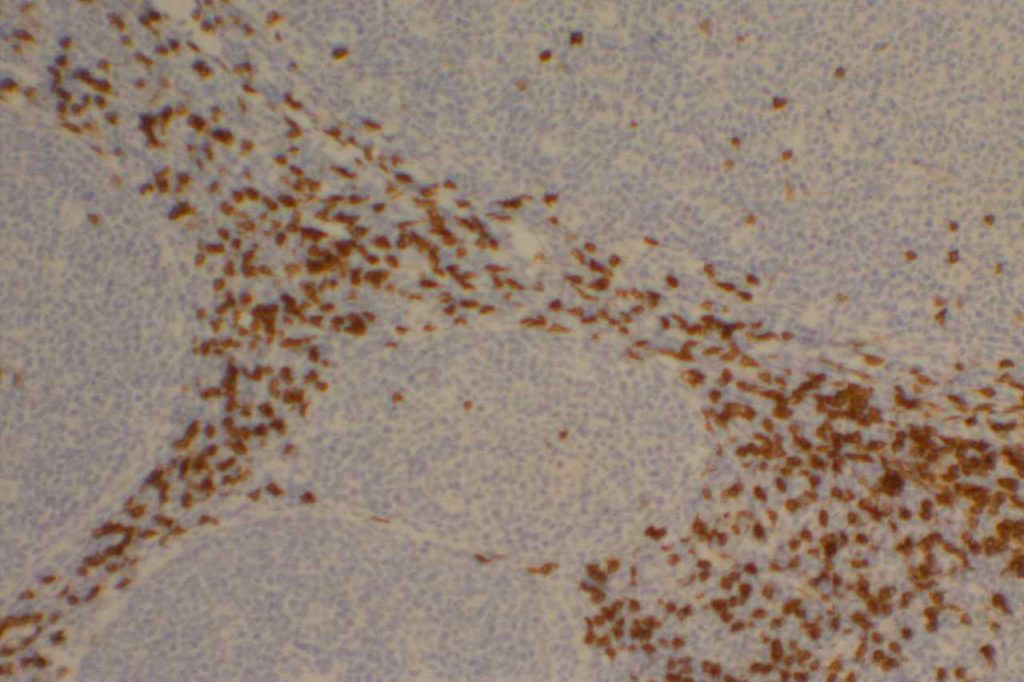
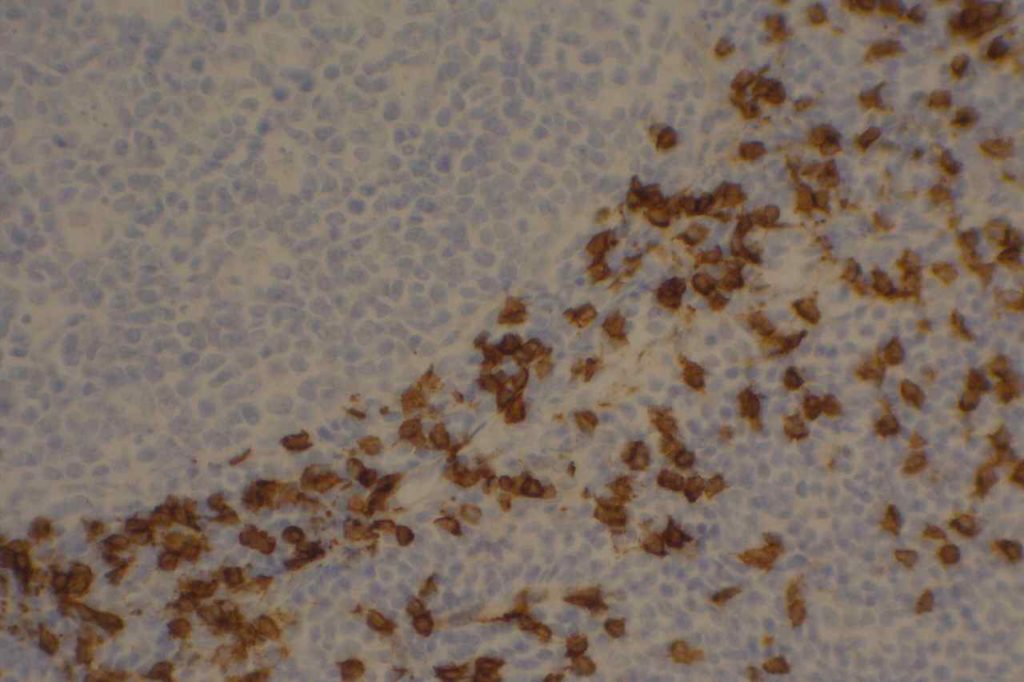
CD8
CD8 is aT-cell marker that is expressed by cytotoxic T-cells (contains perforin and granzyme, which are directly toxic), and stains a sub-set of CD3 positive T-cells. CD3 positive T-cells express either CD4 or CD8. Only thymocytes should express both CD4 and CD8 during a phase of their maturation process. CD8 can be a helpful marker in characterizing T-cell disorders.
T-Cell antigens (usually CD5) can be expressed in B-cell lymphomas, but occasionally other T-cell markers (including CD8) may also be expressed (CD8, up to 3% but flow cytometry). Use of panels (particularly when dealing with T-cell differentiation) is generally more helpful.
Quantification (absolute or relative to CD3 and/or CD4) of CD8+ lymphocytes associated with tumors (stromal infiltration, tumor infiltrating lymphocytes, etc.) is an active area of research. Quantification of such findings has shown prognostic significance (varies based on tumor), and will likely become even more important as treatment regimen begin to more frequently incorporate “immunotherapy” (e.g. PD-L1 inhibitors).
Evaluation of T-cells subsets is also an area of interest in medical conditions such as gluten-sensitive enteropathy and colitis. However, no standard use of T-cell markers is currently a common practice. Though some advocate use in detection of early cases of gluten-sensitive enteropathy (controversial).
Other cytotoxic markers, such as granzyme, TIA-1, and perforin, may be helpful to further characterize CD8 expression lesions/neoplasms to confirm the cytotoxic nature of the cells vs. aberrant expression in a non-cytotoxic lesion.
CD8 Expression
- Dendritic cells of lymphoid origin (dendritic cells of myeloid origin do not express CD8)
- T-cell Lymphomas (CD8+ less common than CD4+)
- Mature T-cells (subset CD4-)
- T-ALL
- T-cell Large Granular Lymphocyte Leukemia
- Dim expression may be seen in endothelial cells
Photomicrographs
References
Carulli G, Stacchini A, Marini A, Ciriello MM, Zucca A, Cannizzo E, et al. Aberrant expression of CD8 in B-cell non-Hodgkin lymphoma: a multicenter study of 951 bone marrow samples with lymphomatous infiltration. Am J Clin Pathol. 2009;132: 186–90; quiz 306. doi:10.1309/AJCPNCOHS92ARWRQ
Paulson KG, Iyer JG, Simonson WT, Blom A, Thibodeau RM, Schmidt M, et al. CD8+ Lymphocyte Intratumoral Infiltration as a Stage-Independent Predictor of Merkel Cell Carcinoma Survival: A Population-Based Study. Am J Clin Pathol. 2014;142: 452–458. doi:10.1309/AJCPIKDZM39CRPNC
An JL, Ji QH, An JJ, Masuda S, Tsuneyama K. Clinicopathological analysis of CD8-positive lymphocytes in the tumor parenchyma and stroma of hepatocellular carcinoma. Oncol Lett. 2014;8: 2284–2290. doi:10.3892/ol.2014.2516
Ortonne N, Buyukbabani N, Delfau-Larue M-H, Bagot M, Wechsler J. Value of the CD8-CD3 ratio for the diagnosis of mycosis fungoides. Mod Pathol. 2003;16: 857–862. doi:10.1097/01.MP.0000084112.81779.BB
Hudacko R, Kathy Zhou X, Yantiss RK. Immunohistochemical stains for CD3 and CD8 do not improve detection of gluten-sensitive enteropathy in duodenal biopsies. Mod Pathol. 2013;26: 1241–1245. doi:10.1038/modpathol.2013.57
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 32-33.
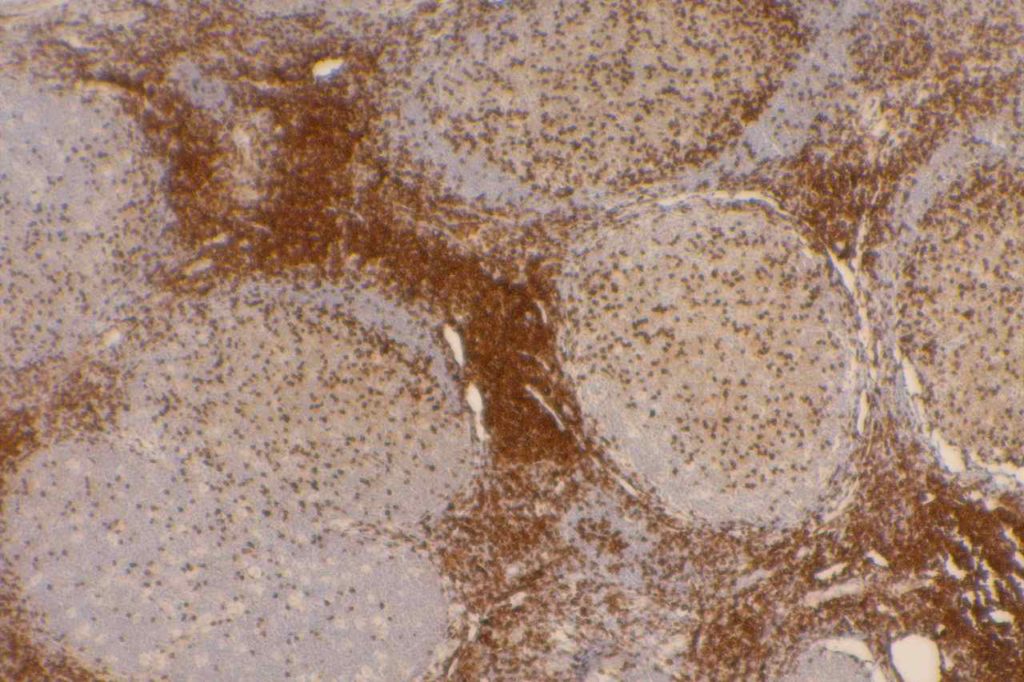
CD7
CD7 is a T-cell marker. It is a specific T-cell marker first expressed early in T-cell development, but often one of the first to be dropped in cases of T-cell lymphoma. However, loss of CD7 expression is not SPECIFIC for lymphoma, as normal benign T-cells may also lose expression. Therefore the IHC findings must always be combined with the morphology when making a diagnosis of T-cell lymphoma. CD7 is typically used as part of a larger T-cell panel including CD2, CD3, CD4, CD5, CD7, and CD8. In challenging cases TCR gene rearrangement studies may also be helpful (specificity is not as good as B-cell gene rearrangement studies).
Aberrant expression of CD7 (and other T- and B-Cell markers) may be seen in acute leukemias (sometimes a helpful finding as a strategy for detecting minimal residual disease by flow cytometry).
CD7 Expression
- Thymocytes
- Mature T-cells
- T-ALL
- Mycosis Fungoides (often drops CD7 expression)
Photomicrographs
References
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 31-32.
Campbell SM, Peters SB, Zirwas MJ, Wong HK. Immunophenotypic diagnosis of primary cutaneous lymphomas: a review for the practicing dermatologist. J Clin Aesthet Dermatol. 2010;3: 21–25.
Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26 Suppl 1: S71–87. doi:10.1038/modpathol.2012.181
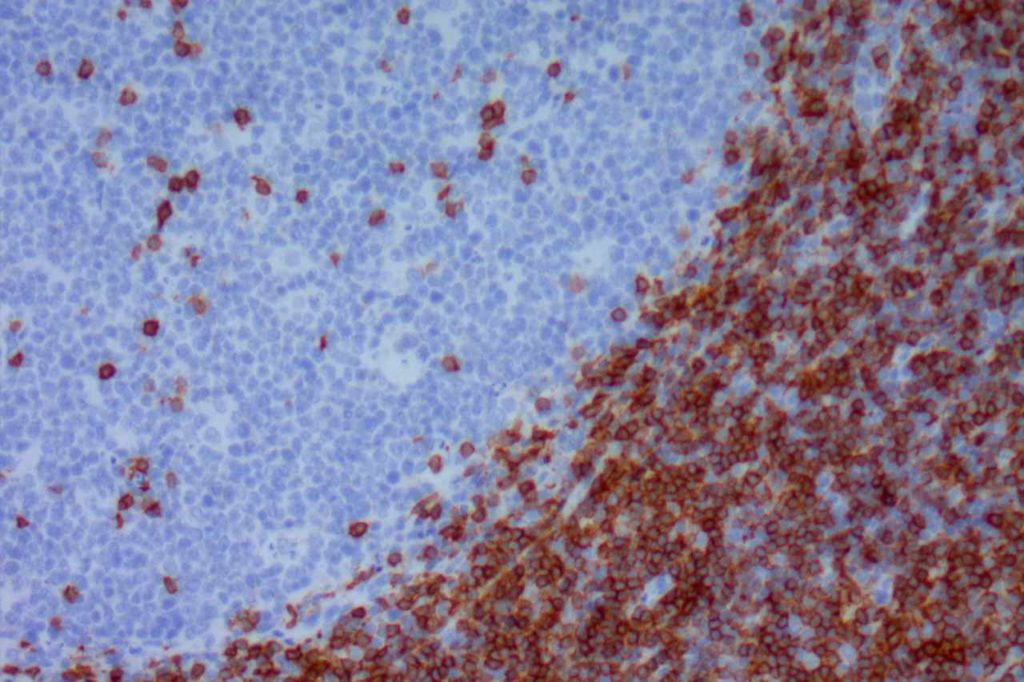
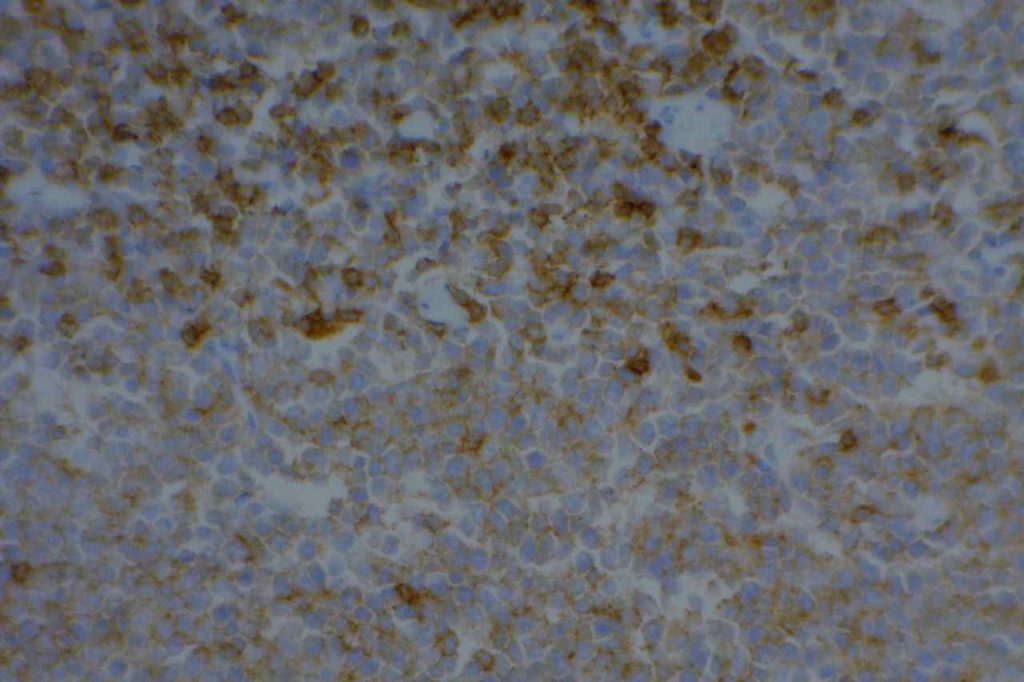
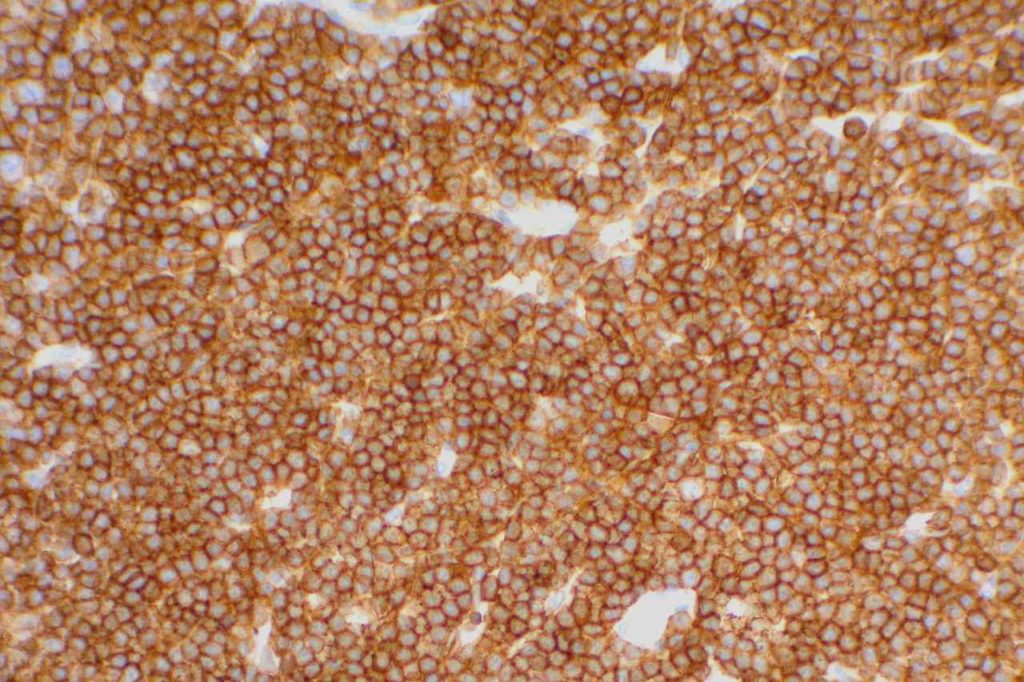
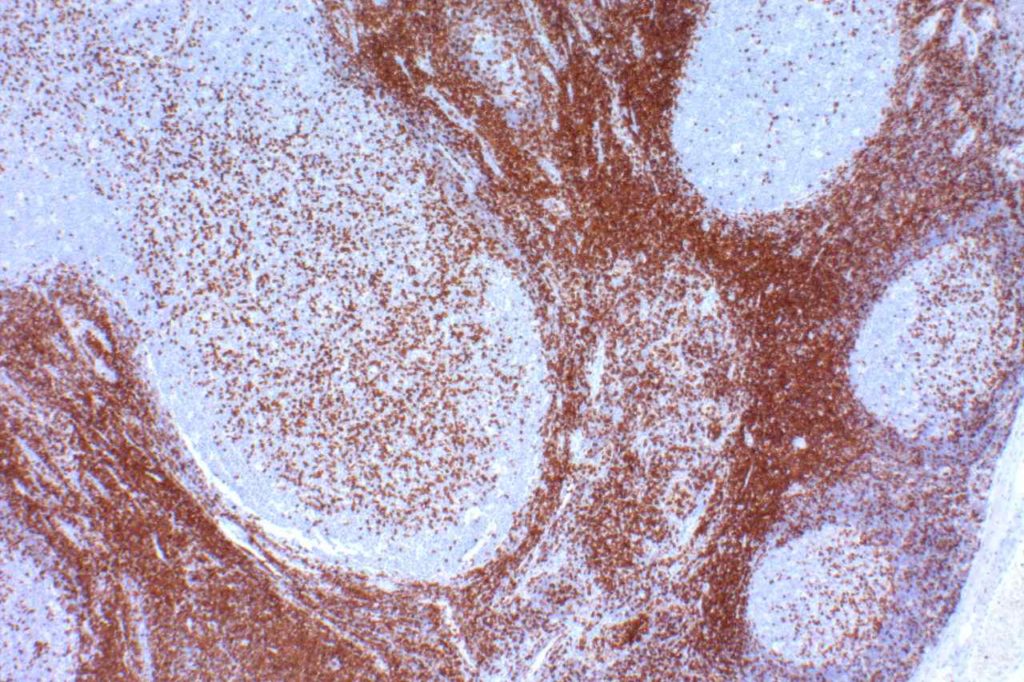
CD5
CD5 is a T-cell marker, although it is the least specific of the group (CD2, CD3, CD4, CD5, CD7, and CD8). CD5 co-expression on B-cells is characteristic of certain lymphomas, specifically CLL/SLL and Mantle Cell Lymphoma. A subset of DLBCLs may also express CD5, although there may be a question as to if that represents de novo lymphoma vs. “transformed” CLL/SLL or Mantle Cell Lymphoma.
The expression level of CD5 will often be less than the expression on background T-cells in cases of CLL/SLL and Mantle Cell Lymphoma, and this expression differentiation may be helpful by both IHC and flow cytometry.
CD5 Expression
- CLL/SLL (often dimmer than background T-cells) – Typically co-express CD5 & CD23. A small portion of cases may be CD5-. LEF1 is a newer marker that is expressed in CLL/SLL and may be helpful for confirming atypical cases of CLL/SLL.
- Mantle Cell Lymphoma – Approximately 12% may not express CD5. SOX11, Cyclin D1 (bcl-1) and FISH for t(11;14) are helpful in atypical cases.
- Nodal Marginal Zone Lymphoma – Up to 8-9% of cases may have co-expression of CD5
- Normal T-cells
- Diffuse Large B-cell Lymphoma (small subset, ~5%) – Associated with more aggressive course, bone marrow involvement, and CNS involvement.
- Lymphoplasmacytic Lymphoma (~5%)
- T-cell Lymphomas
Many different B and T-cell lymphomas have reported CD5, and while it is a useful marker, specificity should be determined with a broader set of markers in addition to the morphology of the lymphoma. Molecular studies may also be helpful.
Photomicrographs
References
Dong HY, Gorczyca W, Liu Z, Tsang P, Wu CD, Cohen P, et al. B-cell lymphomas with coexpression of CD5 and CD10. Am J Clin Pathol. 2003;119: 218–230. doi:10.1309/U98A-DVKU-C26R-2RJA
Gao J, Peterson L, Nelson B, Goolsby C, Chen Y-H. Immunophenotypic variations in mantle cell lymphoma. Am J Clin Pathol. 2009;132: 699–706. doi:10.1309/AJCPV8LN5ENMZOVY
Dalton RR, Admirand JH, Medeiros LJ. Small Lymphocytic Lymphoma. Pathology Case Reviews. 2004;9: 7.
Jaso JM, Yin CC, Wang SA, Miranda RN, Jabcuga CE, Chen L, et al. Clinicopathologic Features of CD5-Positive Nodal Marginal Zone Lymphoma. Am J Clin Pathol. 2013;140: 693–700. doi:10.1309/AJCPEMVXES72DUIF
Went P, Zimpfer A, Tzankov A, Dirnhofer S. CD5 expression in de novo diffuse large B-cell lymphomas. Annals of Oncology. 2009;20: 789–790. doi:10.1093/annonc/mdn793
Cook JR. Nodal and leukemic small B-cell neoplasms. Mod Pathol. 2013;26 Suppl 1: S15–28. doi:10.1038/modpathol.2012.180
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 27.
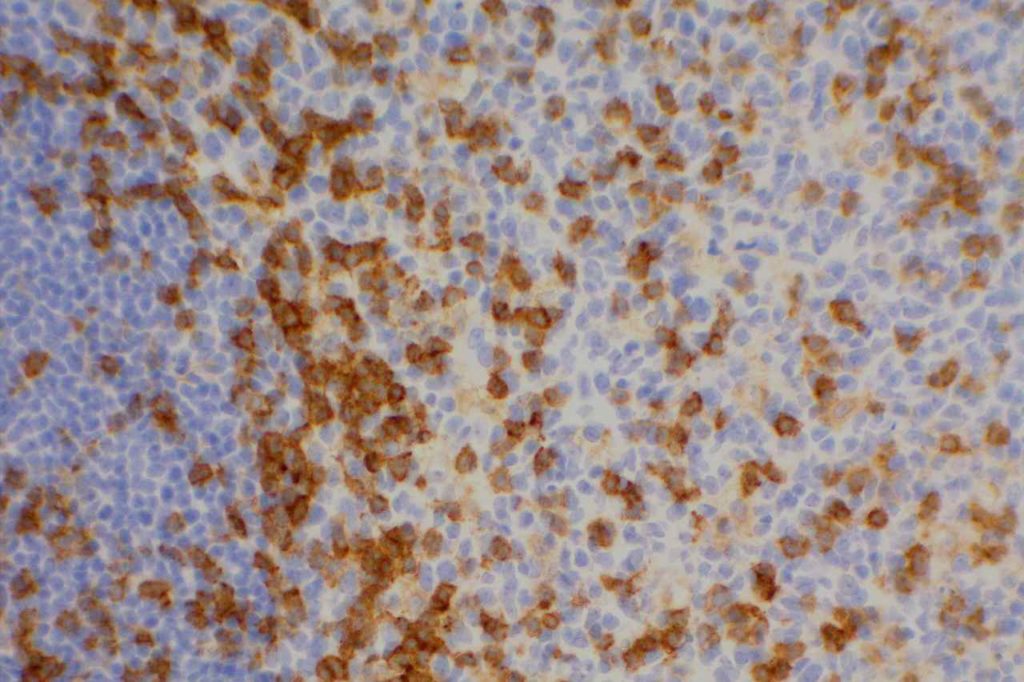
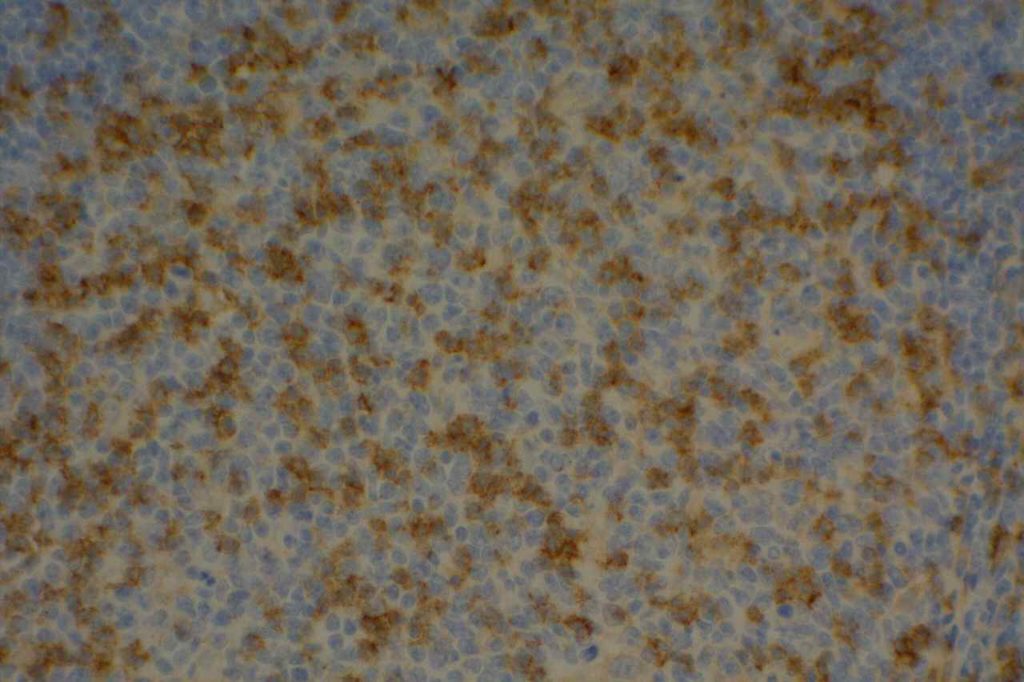
CD4
CD4 is a glycoprotein on the surface of a subset of T-cells, which defines the T-helper subset. T-helper cells help to activate T-cell and B-cell immune response/proliferation in response to a foreign antigen. CD4 is the target/receptor of the HIV virus.
CD4 stains a subset of CD3 positive T-cells (T-helper cells). CD4 is expressed by thymocytes (80-90%), macrophages, Langerhans cells, and dendritic cells. Many T-cell leukemia/lymphomas, blastic plasmacytoid dendritic cell neoplasms, and a sub-set of AMLs will express CD4.
Negative Staining: NK cells, B-cell lymphoma, enteropathy associated T-cell lymphoma, epidermotropic cutaneous T-cell lymphoma, hepatosplenic alpha/beta and gamma/delta lymphoma, Hodgkin lymphoma, and T cell lymphoma with cytotoxic phenotype.
CD4 Expression
- Thymoctye subsets
- T-helper cells
- Monocytes
- Macrophages
- CD4+/CD56+ hematodermic neoplasm
- Dendritic cells (can express CD4 and CD8)
- T-cell Lymphomas (CD4+ more common than CD8+)
- Histiocytic disorders
- Subset of AMLs
CD4 is typically used as part of a T-cell panel to identify aberrant expression or loss of expression in cell populations of interest. Given the somewhat lack of specificity of the marker, it should be only interpreted within the context of the differential diagnosis and expected reactivity pattern(s).
By flow cytometry, low levels of CD4 expression has been demonstrated on erythroid precursor cells. This probably lacks diagnostic significance (particularly IHC), but may have implications in HIV where CD4 is the receptor the virus is mediated through.
Photomicrographs
References
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 24-25.
Cleveland RP, Liu YC. CD4 Expression by erythroid precursor cells in human bone marrow. Blood. 1996;87: 2275–2282.
Herling M, Jones D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127: 687–700. doi:10.1309/FY6PK436NBK0RYD4
CD3
CD3 is the most common T-cell marker used. It is considered a “pan T-cell marker”, and shows excellent specificity. It also marks NK cells (CD3 epsilon cytoplasmic 56%), mast cells, variable thymic B-cells (50%), and Langerhans cells. 80% of T-cell lymphomas, NK lymphoma (cytoplasmic, not membraneous), lymphomatous papulosis, and pre-T ALL mark with CD3.
CD3 expression is first found in pro-thymocytes (peri-nuclear), which develops into cytoplasm expression, and finally is expressed on the cell membrane with absence of cytoplasmic staining. This expression pattern parallels T-cell development from pro-thymocytes to common thymocytes and finally into medullary thymocytes.
Negative Staining
- Most B-cell lymphomas
- Aberrant loss of CD3 may be seen in some cases of mycosis fungoides (MF)
- Anaplastic large cell lymphoma (ALCL), and AILT. In fact, CD3 is the least sensitive T-cell marker for ALCL.
CD3 Expression
- Pan T-cell Marker
- NK cells (cytoplasmic)
- T-cell Lymphomas
- NK/T-cell Lymphoma (nasal type), variable expression
- T-ALL (subset)
Clinical Significance
Interestingly, muromonab (OKT3®) CD3 is a monoclonal drug, which targets and blocks the function of the CD3 receptor. It is used in the treatment of acute allograft rejection in renal transplants.
Photomicrographs
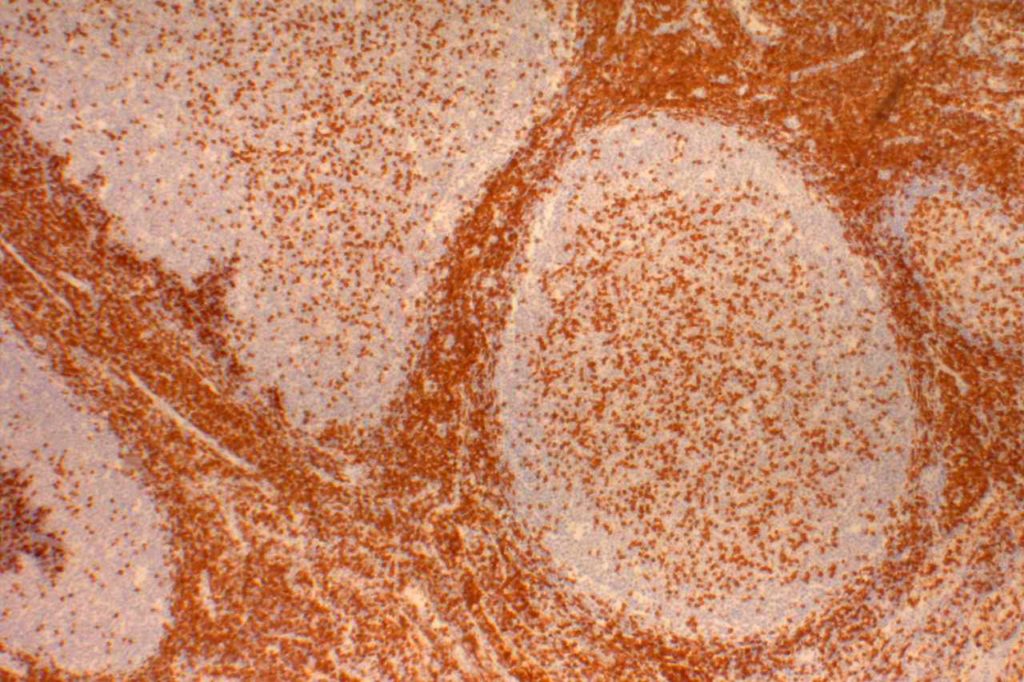
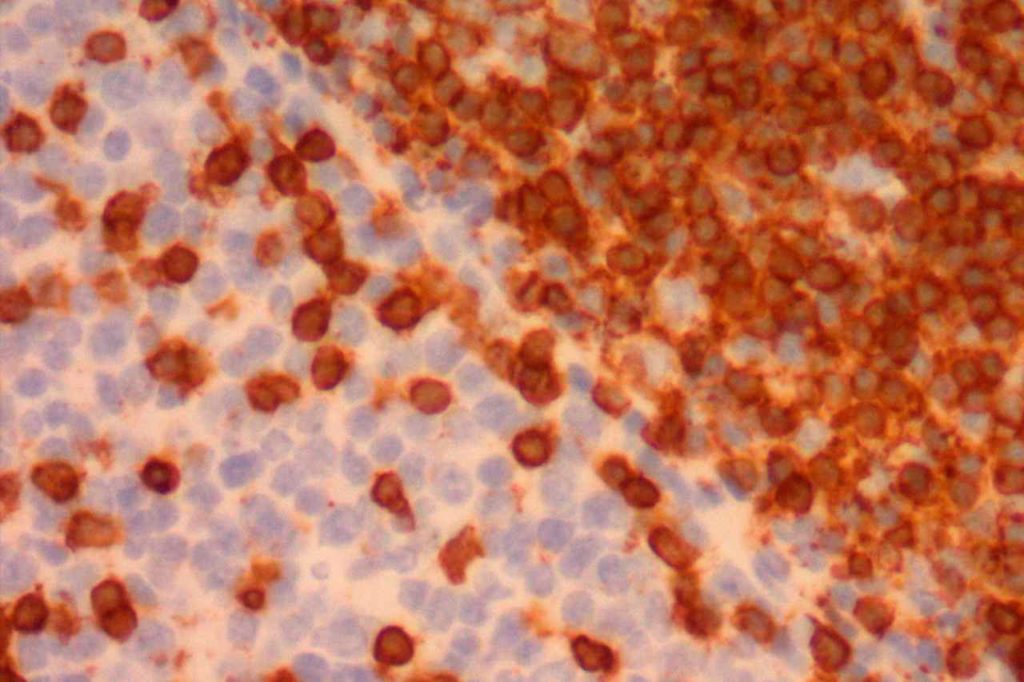
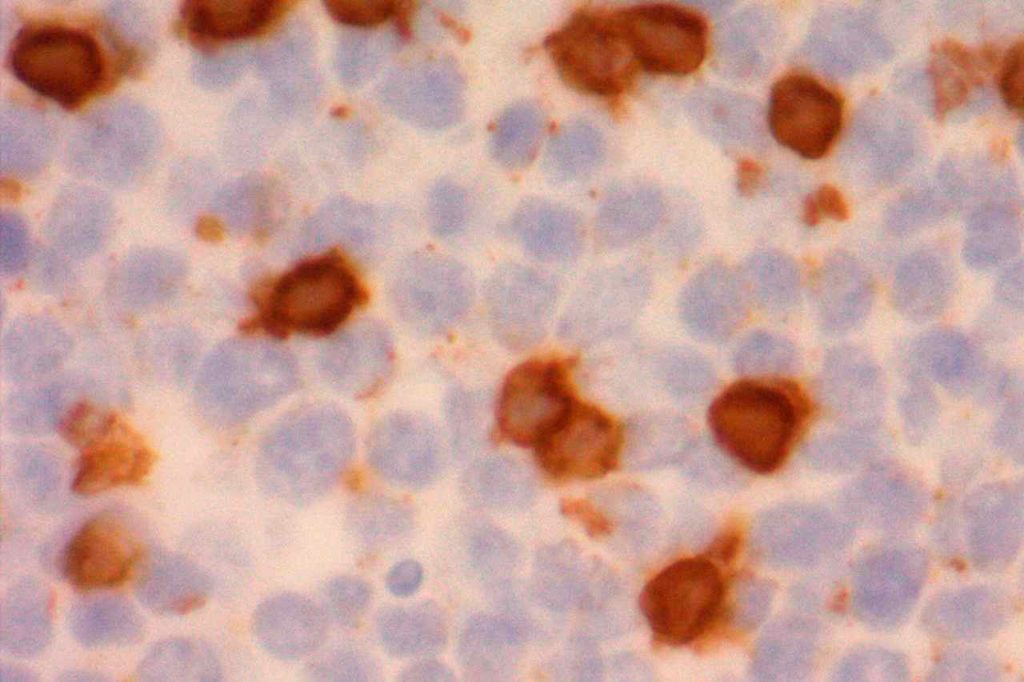
References
Kuby, Janis; Kindt, Thomas J.; Goldsby, Richard A.; Osborne, Barbara A. (2007). Kuby immunology. San Francisco: W.H. Freeman. ISBN 1-4292-0211-4.
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 17-23.
Benekli M, Hahn T, Williams BT, Cooper M, Roy HN, Wallace P, et al. Muromonab-CD3 (Orthoclone OKT3), methylprednisolone and cyclosporine for acute graft-versus-host disease prophylaxis in allogeneic bone marrow transplantation. Bone Marrow Transplant. 2006;38: 365–370. doi:10.1038/sj.bmt.1705450
BioRAD. The T Cell Marker, CD3 Antigen and Antibodies. Mini Review. Accessed 9-6-17.
Dahlin JS, Hallgren J. Mast cell progenitors: origin, development and migration to tissues. Mol Immunol. 2015;63: 9–17. doi:10.1016/j.molimm.2014.01.018
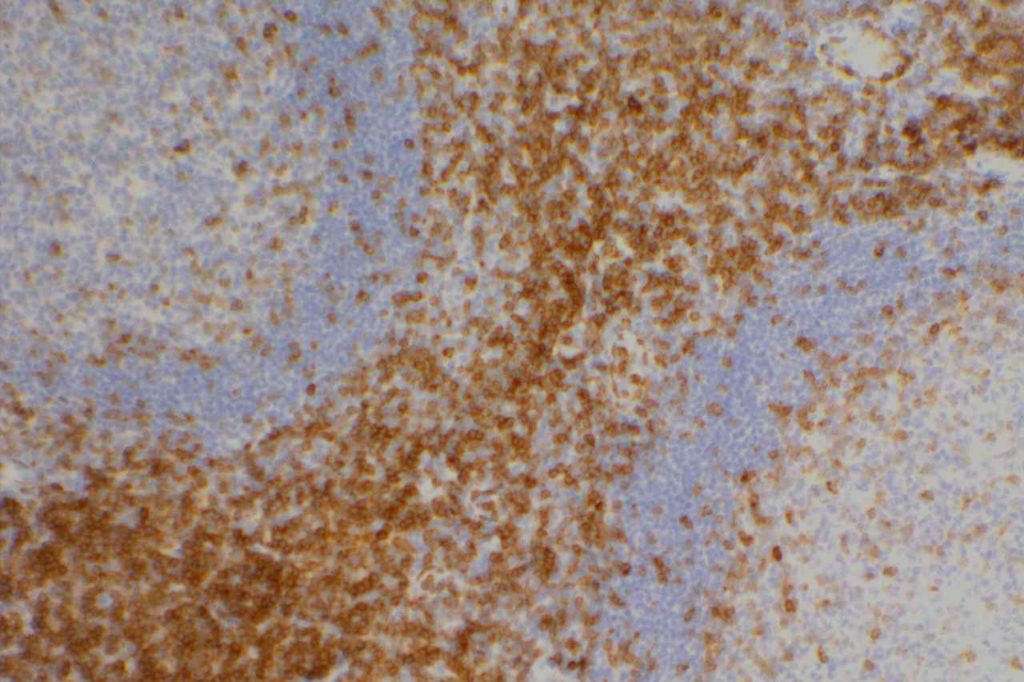
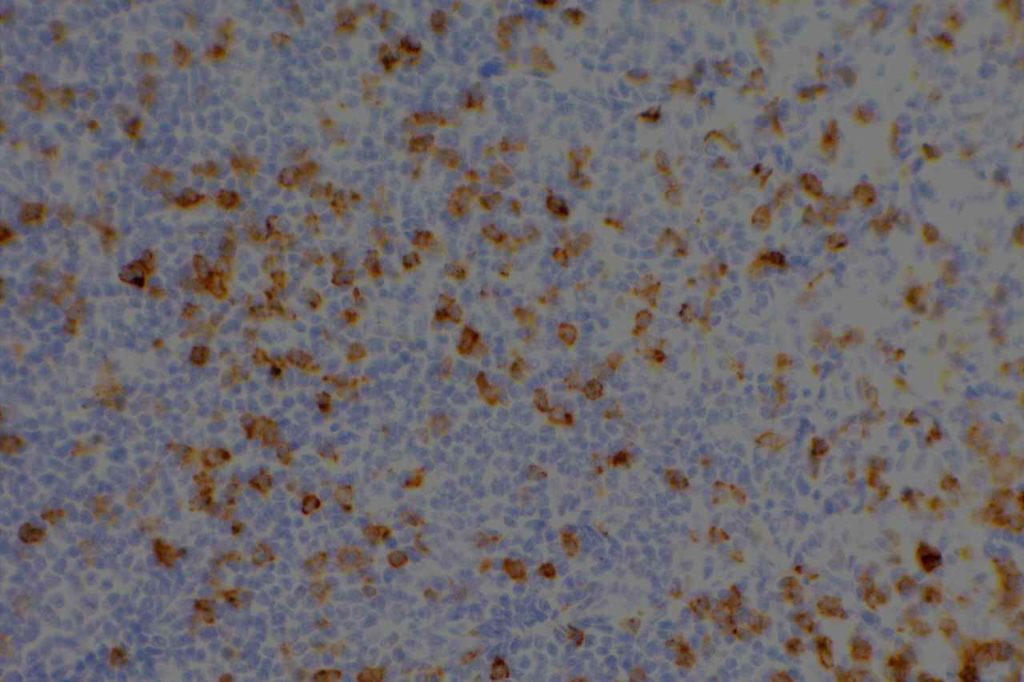
CD2
CD2 is a T-cell and NK cell (80-90%) marker. It also stains mast cells in mastocytosis disorders, and is variable in thymic B-cells (~50%). Some cases of AML will express CD2. CD2 does NOT stain B-cells, basophils, and mast cells in non-mastocytosis disorders. CD2 is one of the earliest T-cell markers.
Practically, CD2 is most often used as part of a T-cell panel to detect T-cell differentiation in a lymphoma of uncertain or presumed T-cell origin. CD3 is the most commonly used T-cell marker. Other T-cell markers include: CD3, CD4, CD5, CD7, and CD8. When evaluating an abnormal T-cell process it is often necessary to do a T-cell panel to look for aberrant antigen expression loss.
CD2 Expression
- T-cells (all)
- NK cells (80-90%)
- Thymic B-cells (~50%)
- Mast Cell Disorders
- AML (Subset)
Reactivity pattern: Membranous and cytoplasmic
Photomicrographs
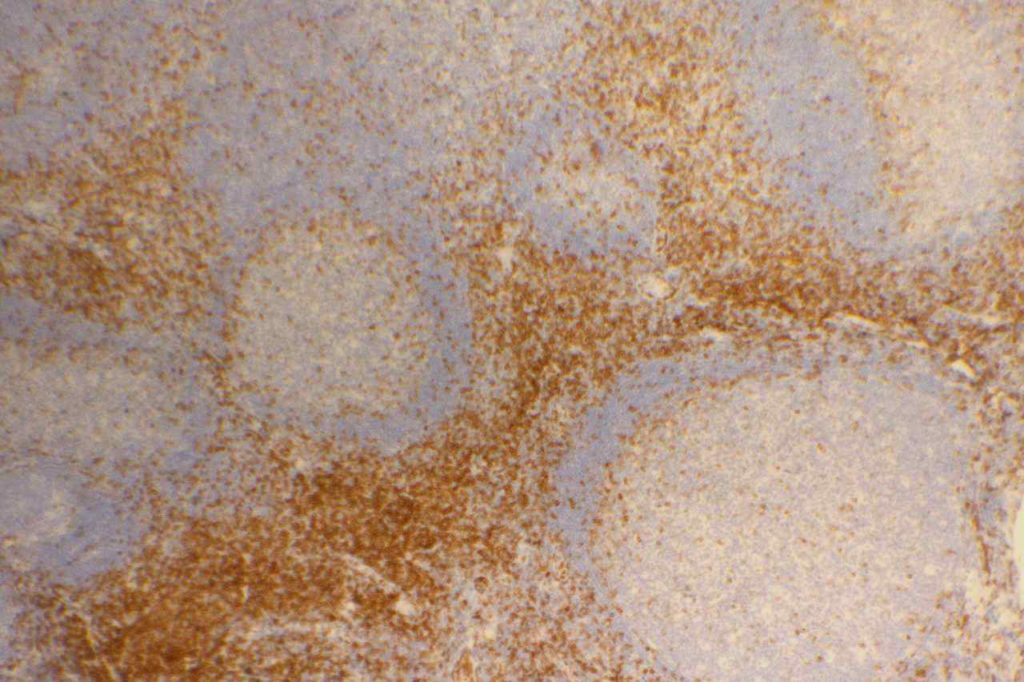
References
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 16-17.
Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. 1987.
Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today. 1996;17: 177–187.