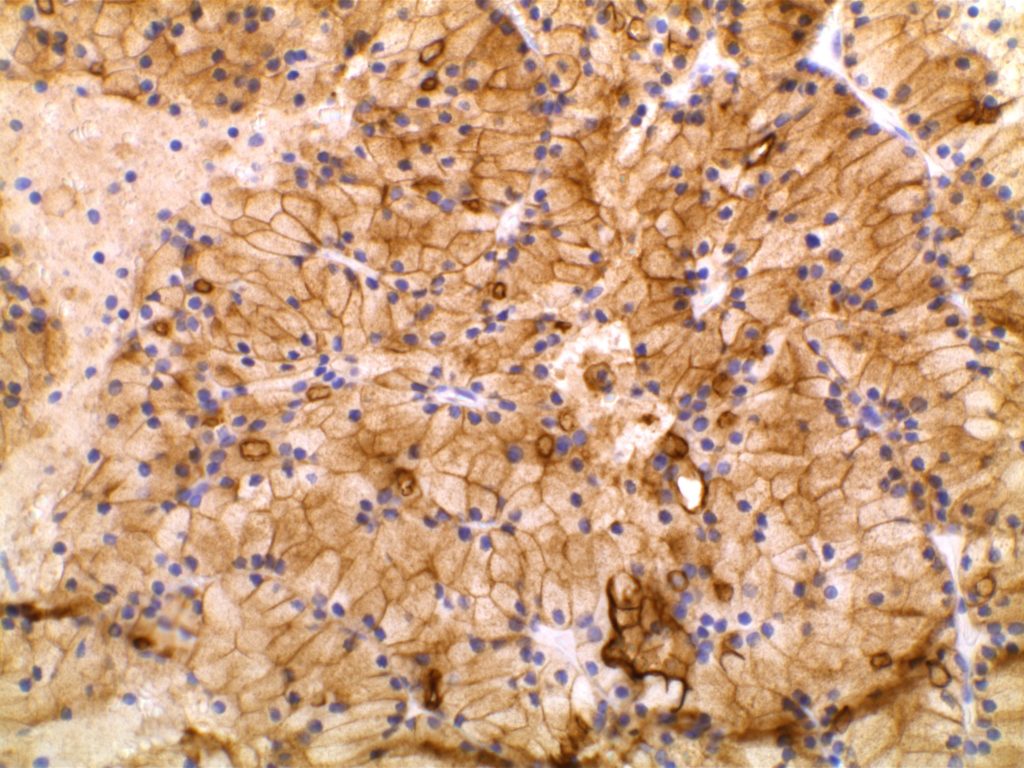
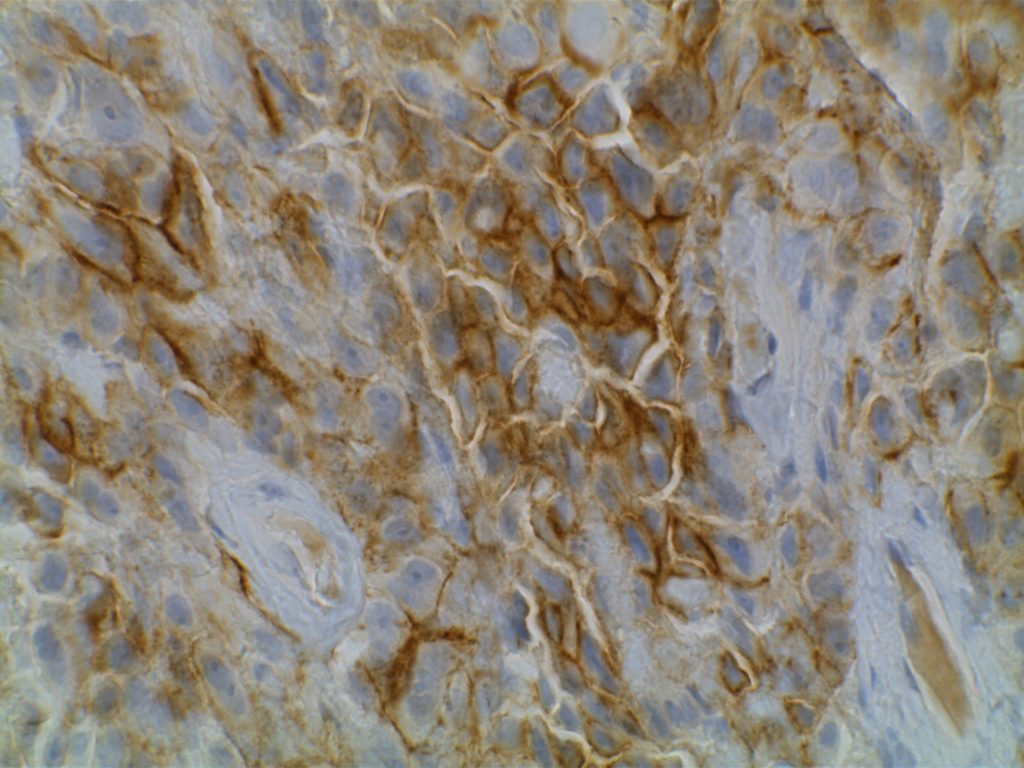
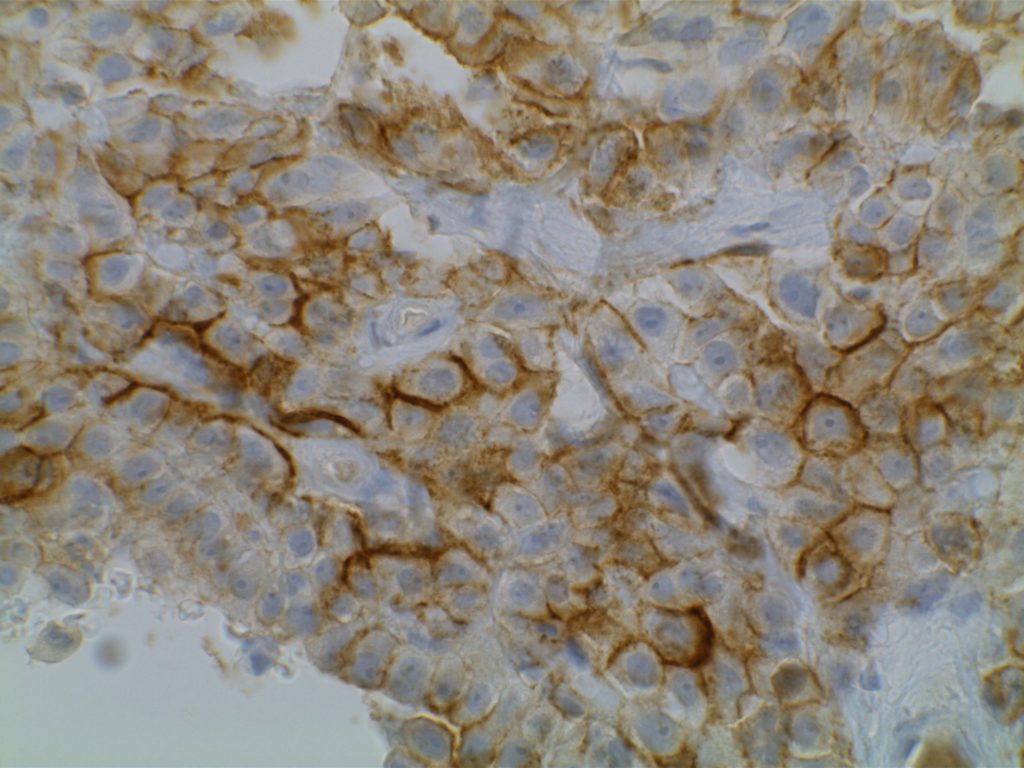
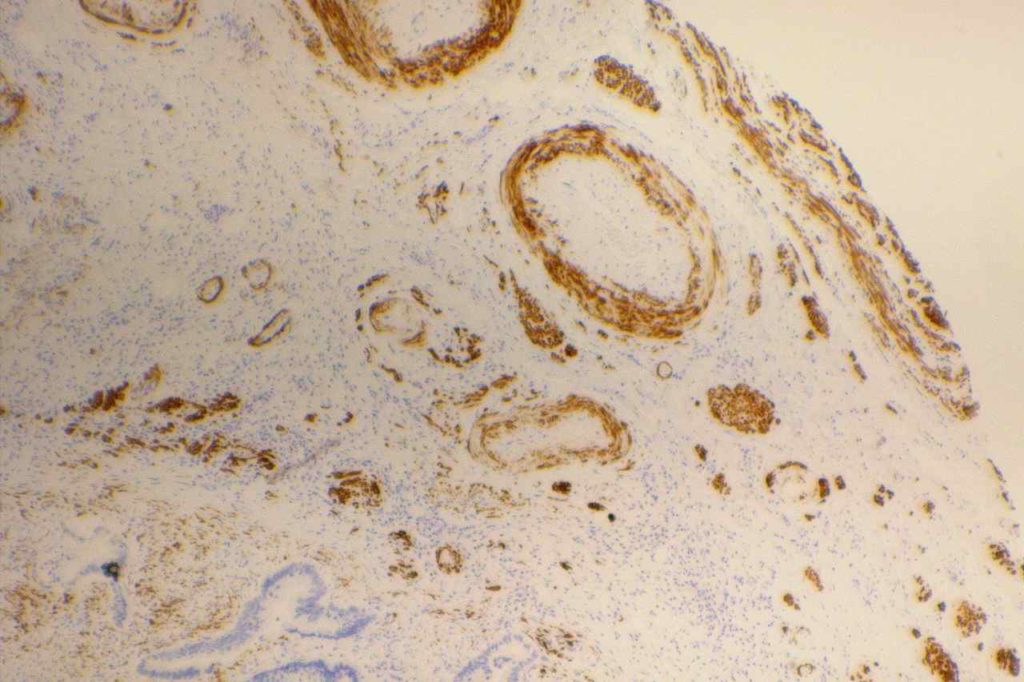
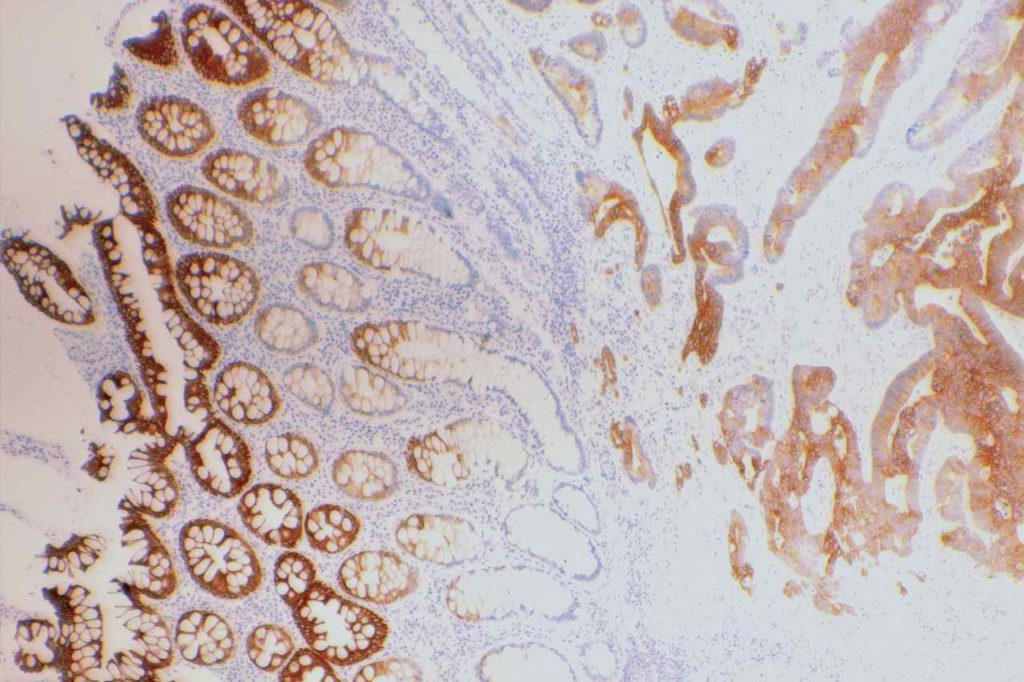
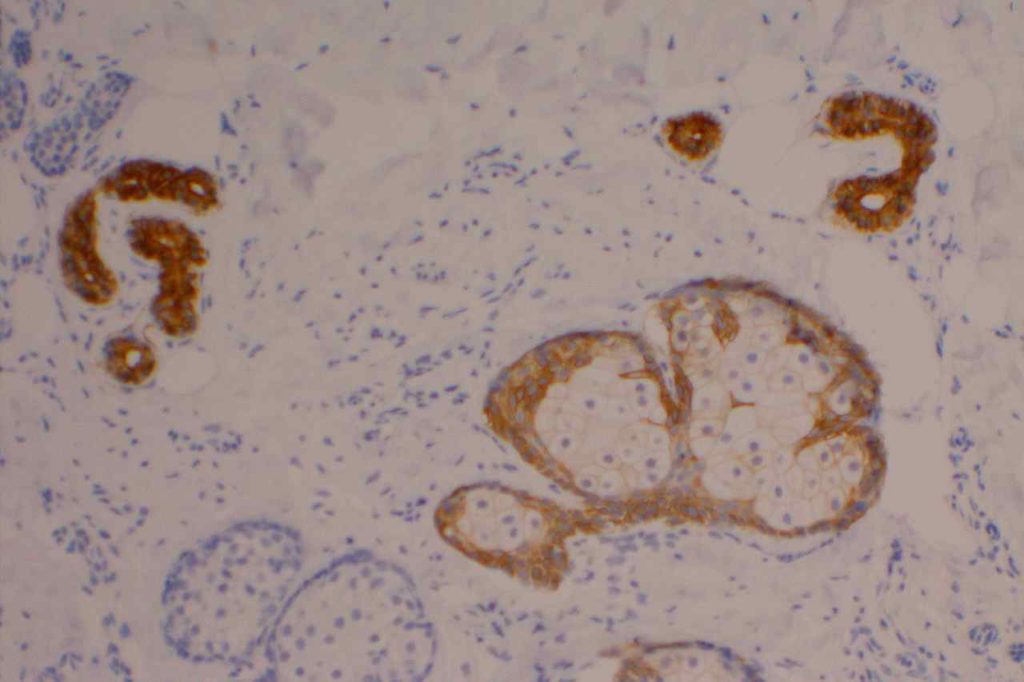
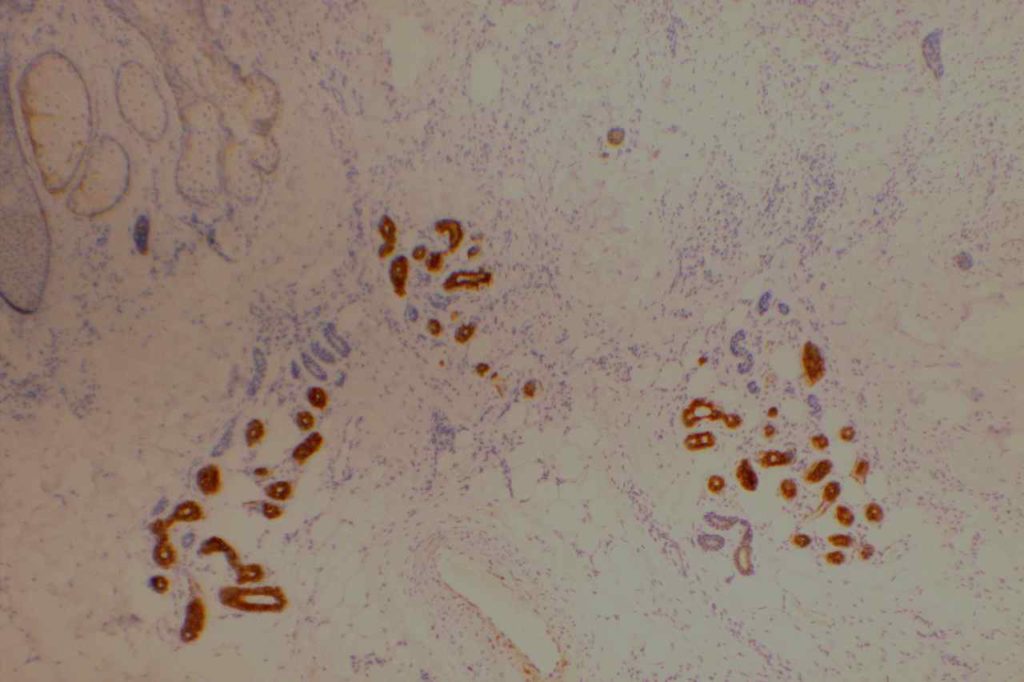
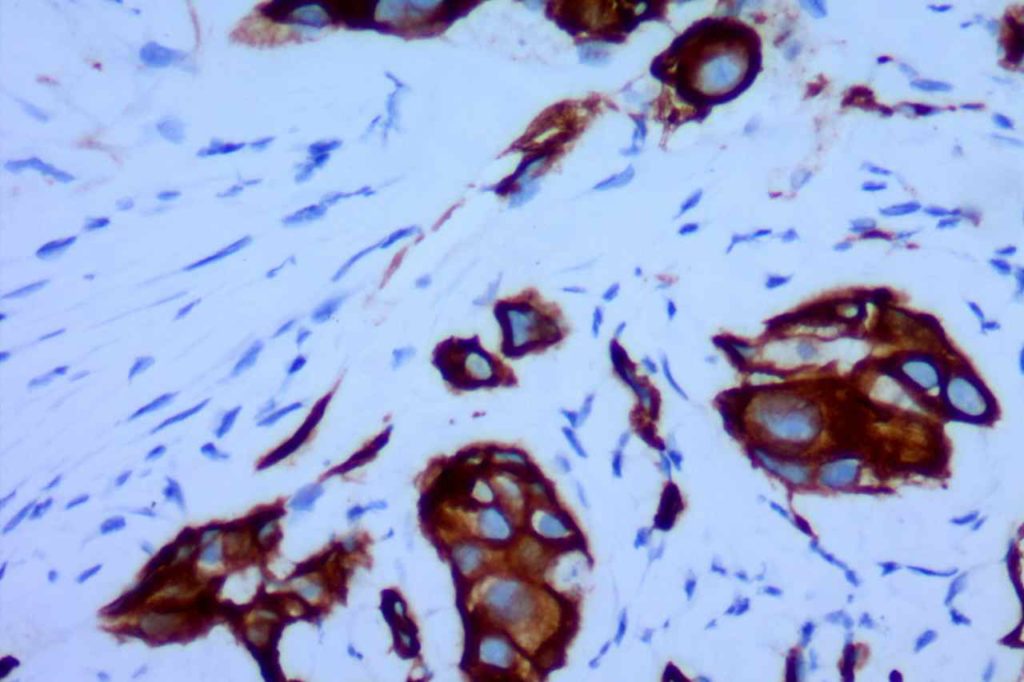
GATA-3 is a member of a subfamily of zinc finger transcription proteins, which has been found to be highly expressed in breast (>90%) (especially lunimal A breast carcinomas) and urothelial carcinomas (>80%). Current evidence suggests that this marker has a better balance of sensitivity and specificity for breast carcinoma than GCDFP-15 and mamaglobin (MGB). Especially, in the setting of ER-negative tumors. GATA-3 use should be considered as part of IHC panels in the setting of carcinoma of unknown primary site. (Lin, et al) Mccluskey, et al. found high GATA-3 expression to be associated with favorable survival and relapse free course in advanced cases.
GATA-3 may also have a role in the evaluation of possible primary bladder tumors. As with most IHC markers, there are very few “silver bullets,” and interpretation should take into consideration the clinical-radiologic context along with the known performance characteristics of the antibody.
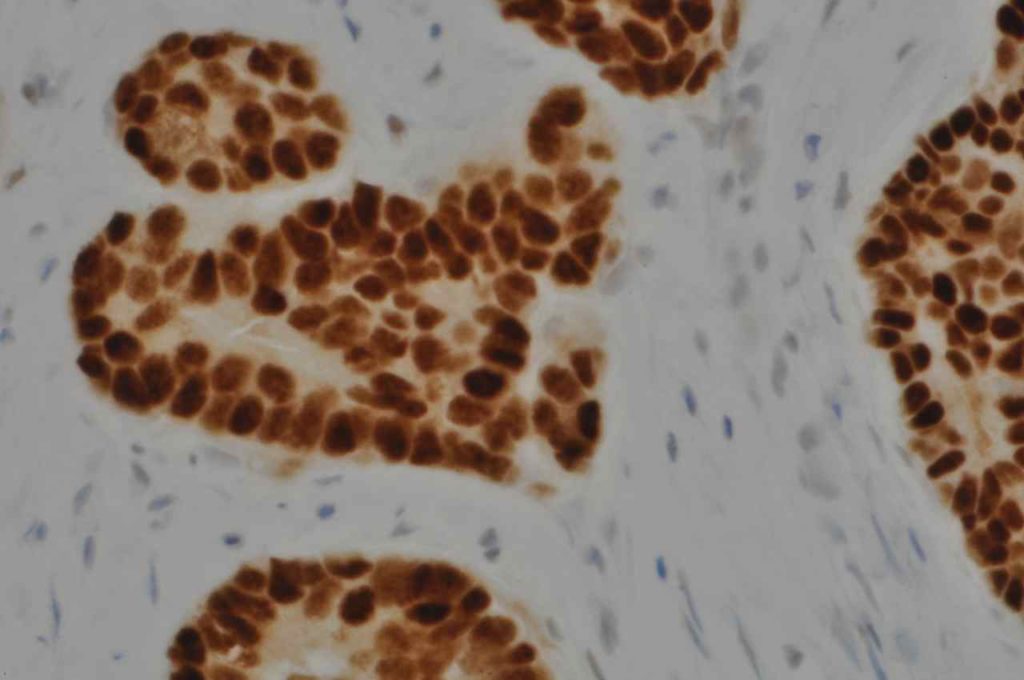
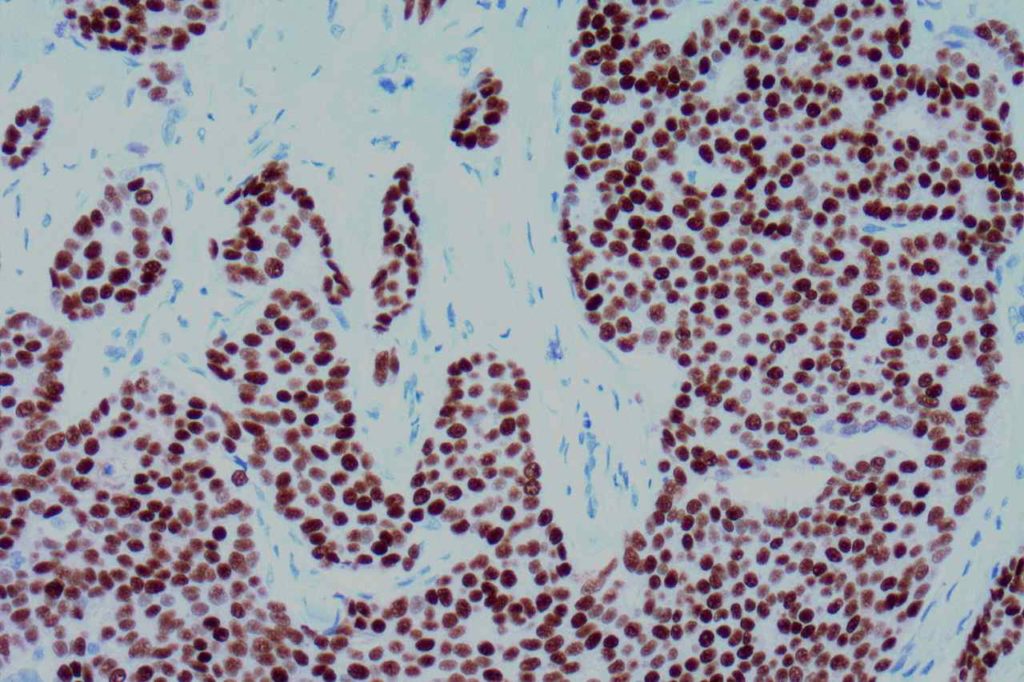
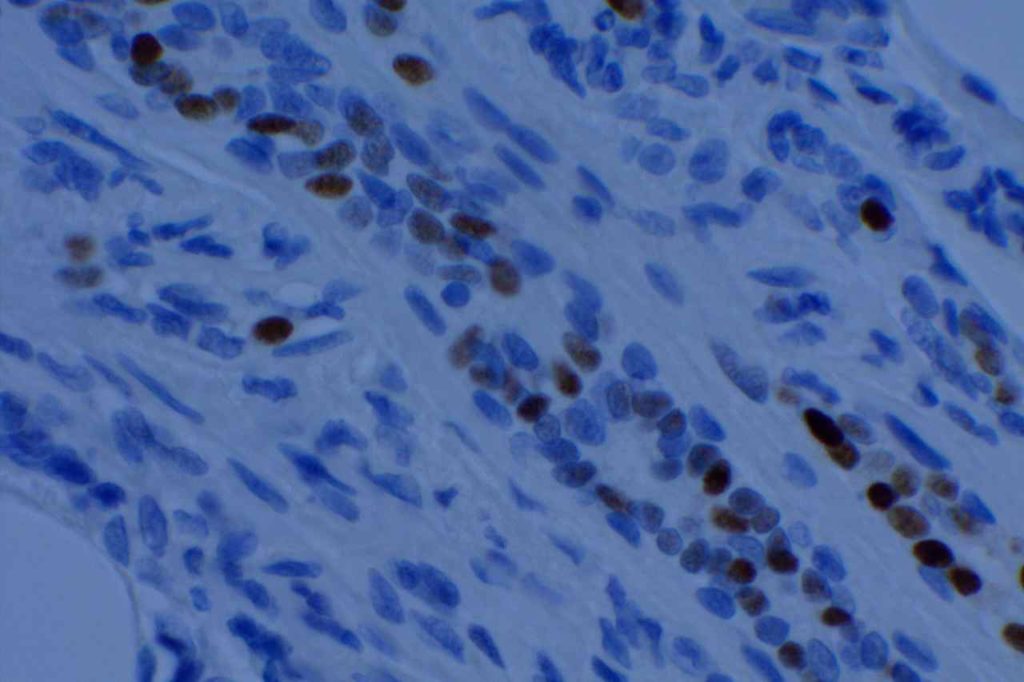
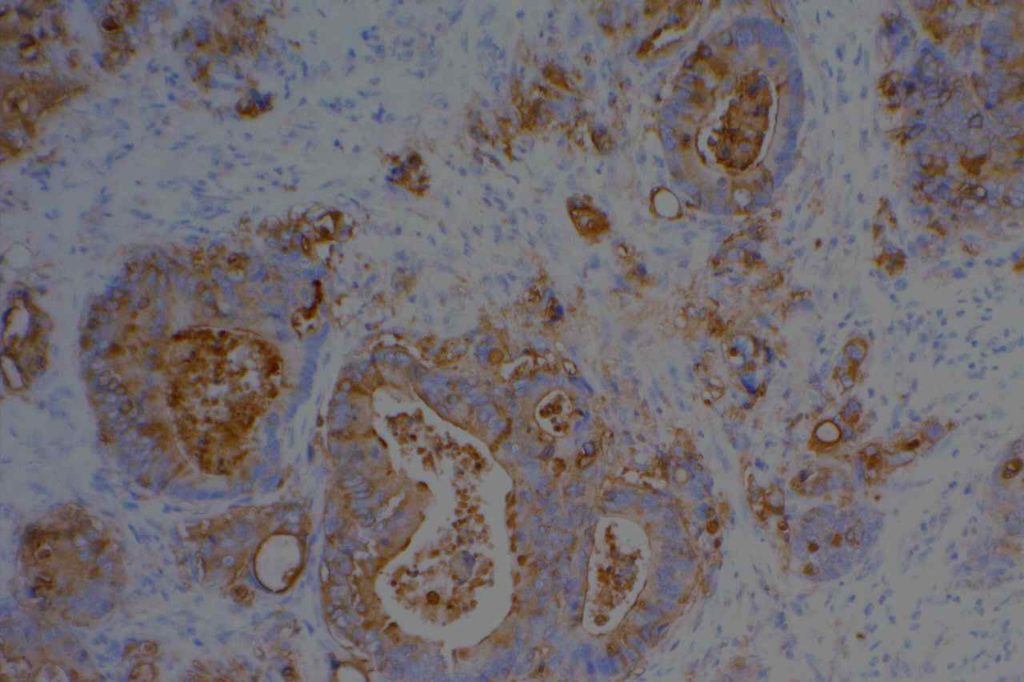
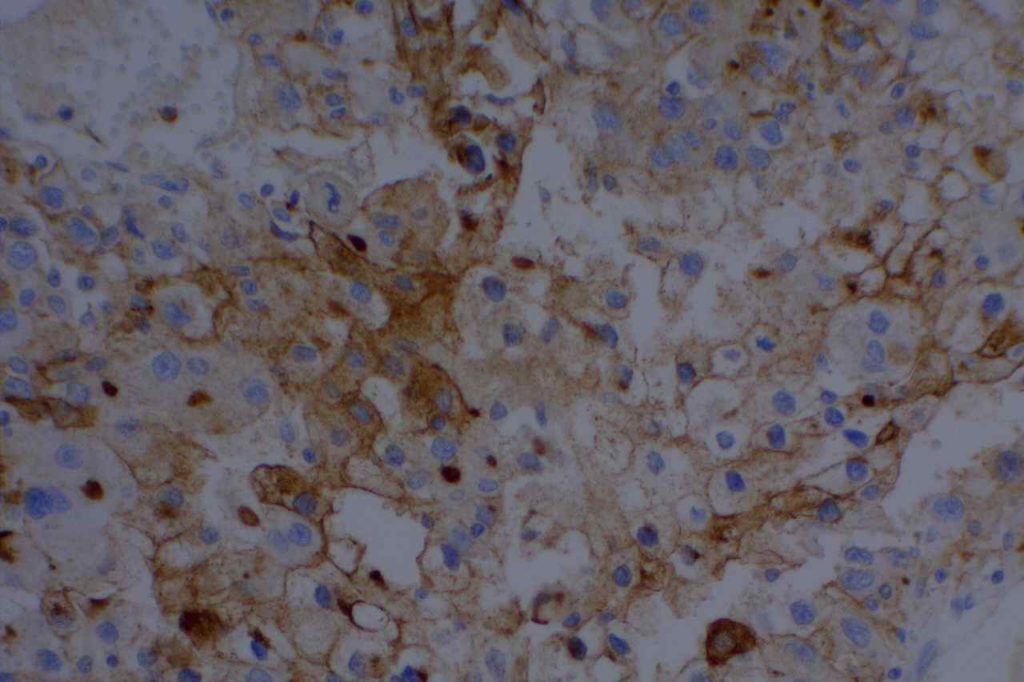
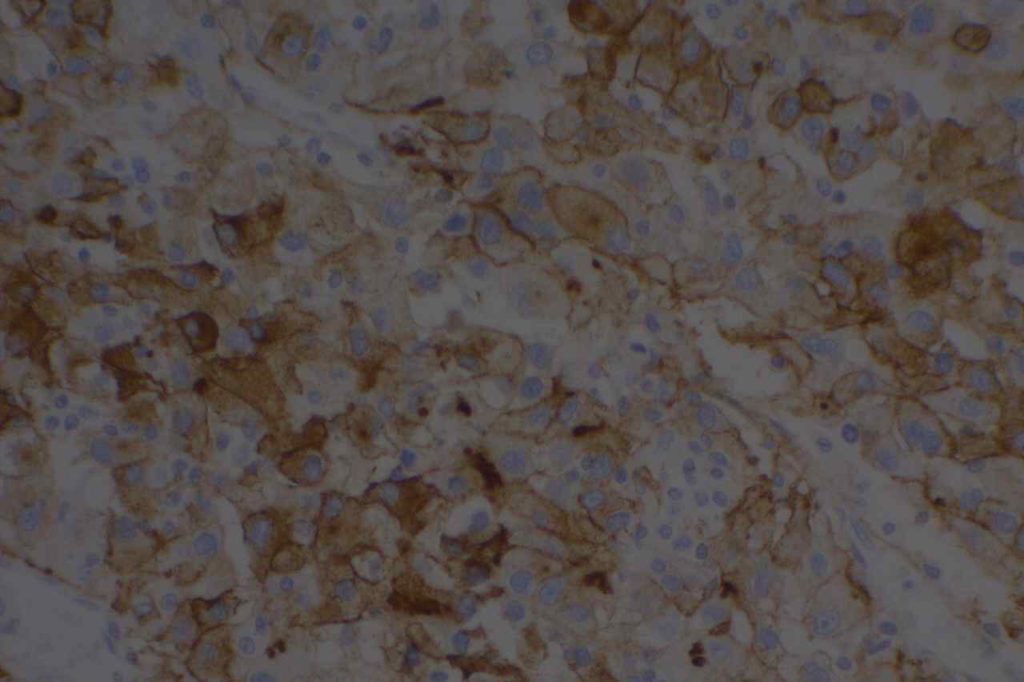
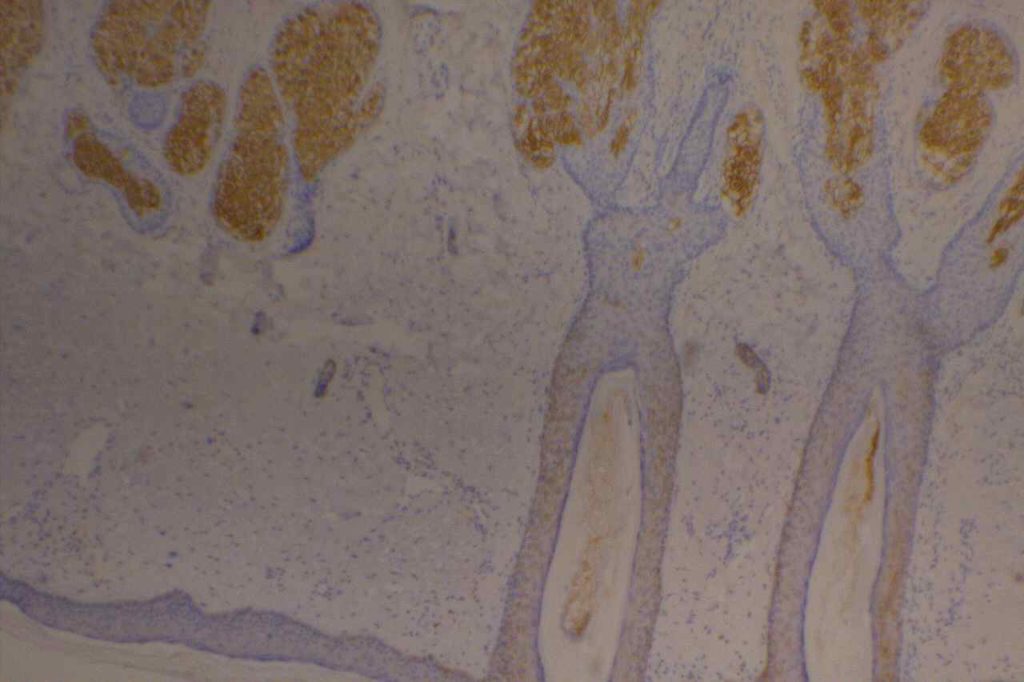
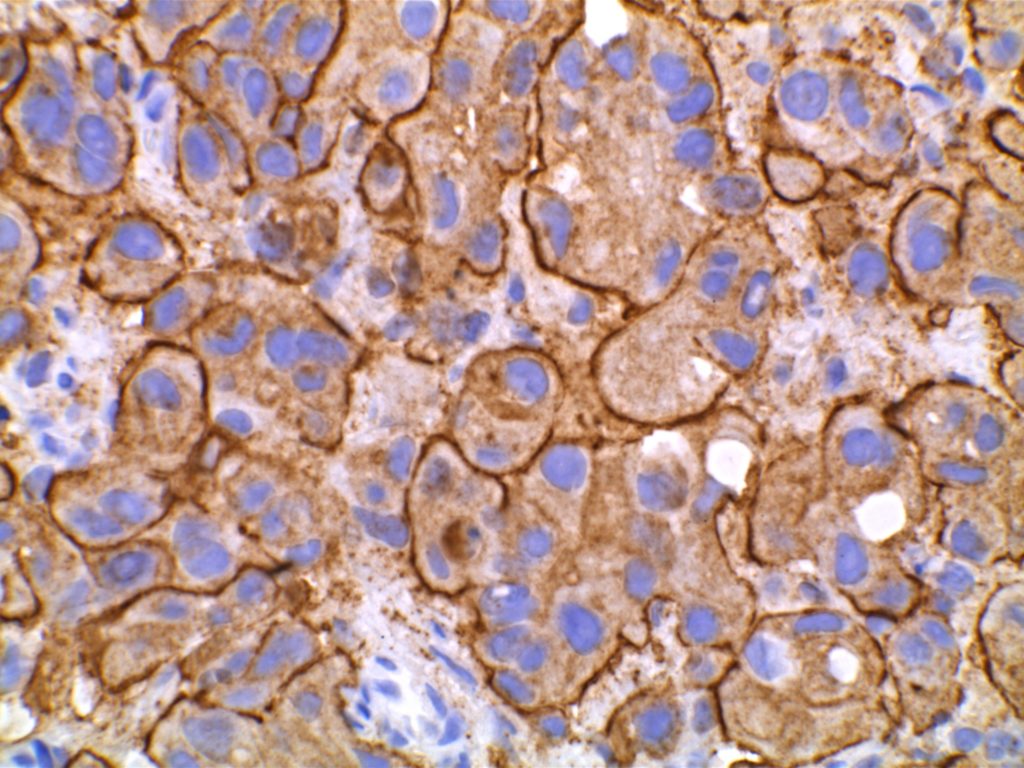
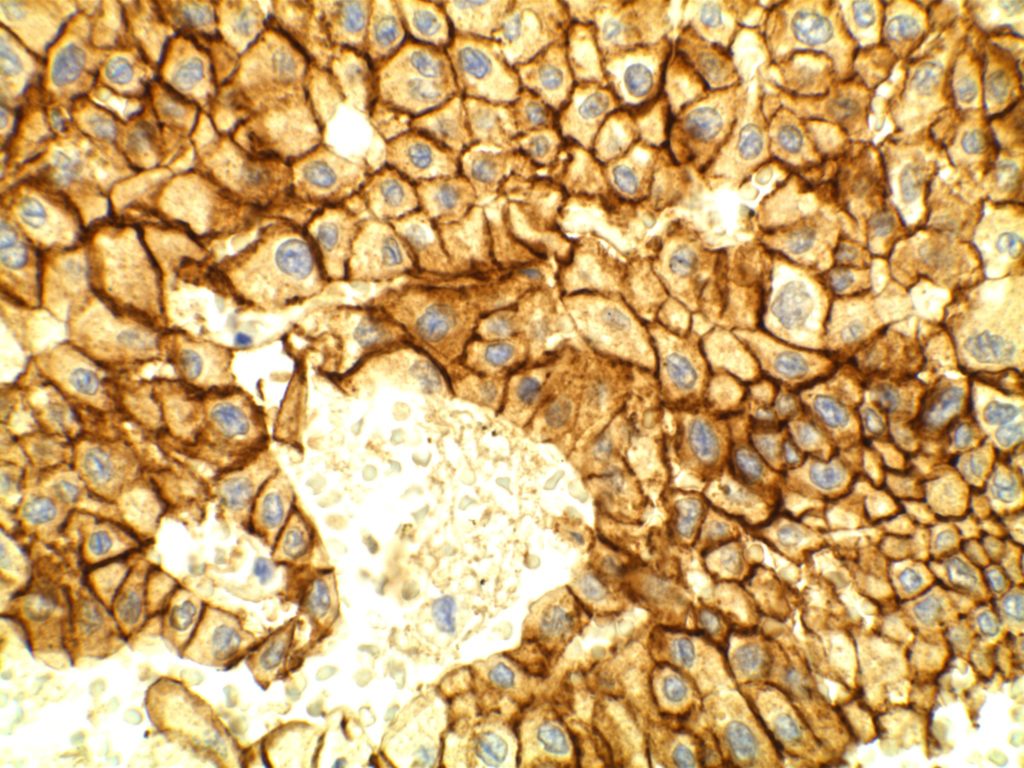
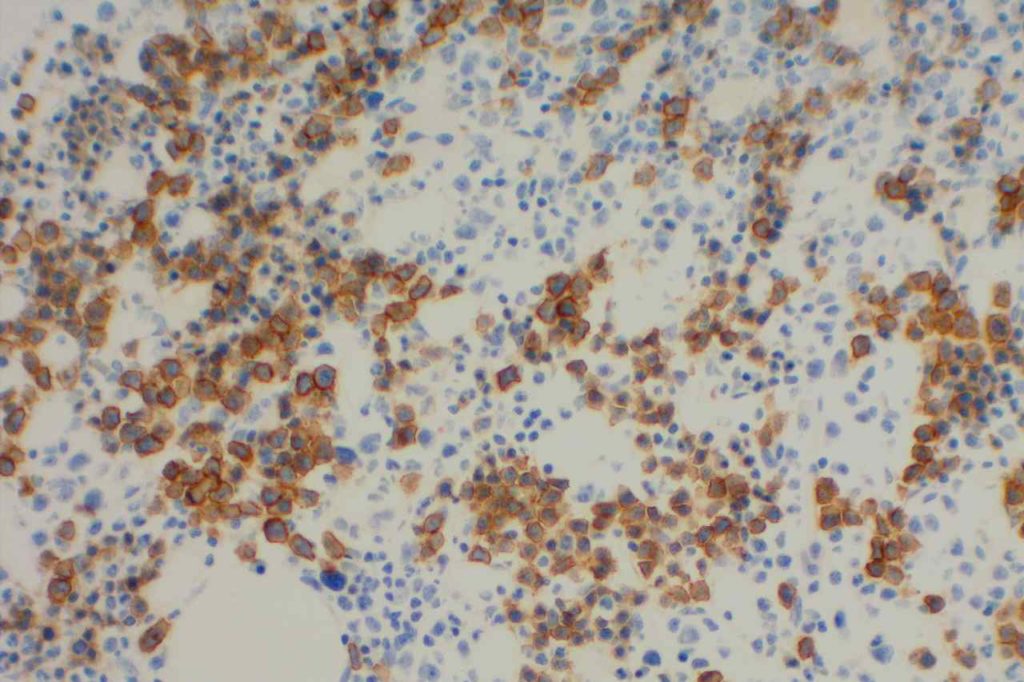
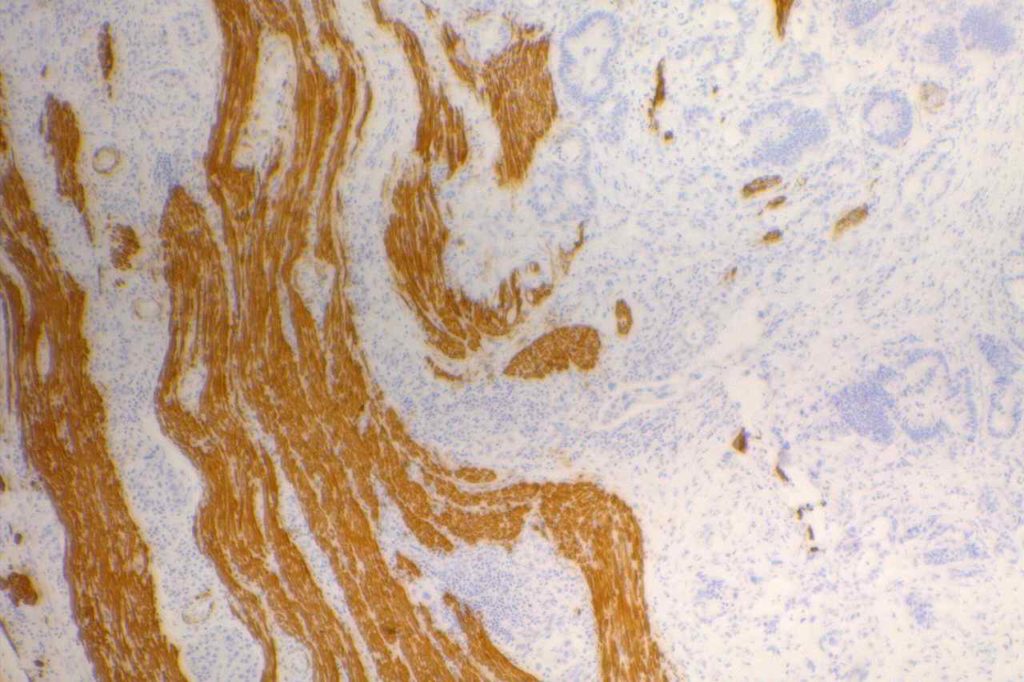
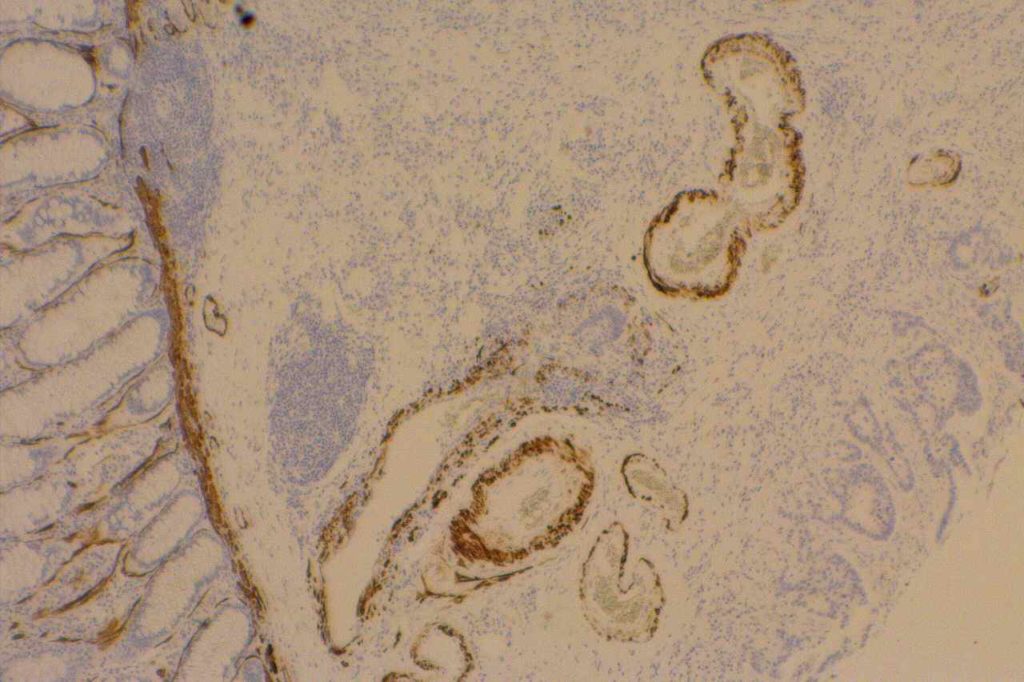
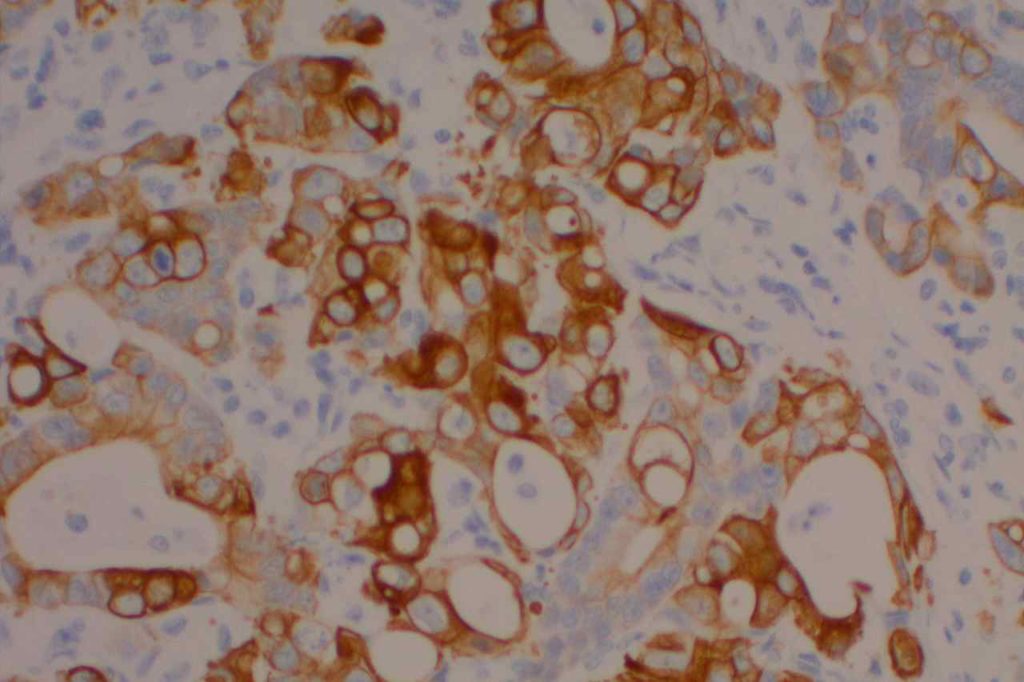
The expression pattern is nuclear, which is typically moderate to strong and diffuse. Some variability of the sensitivity of GATA-3 in breast carcinomas has been noted in the literature, and confirmation of one’s assay compared to the medical literature is recommended during the validation process. The following tables show data from multiple papers in the pathology literature.
Liu, et al (Biocare Medical, Concord, CA)
|
Tumor
|
GATA3
|
GCDFP15
|
MGB
|
|
Breast Carcinoma
|
94%
|
35-55%
|
65-70%
|
|
ER-negative breast ca.
|
69%
|
15%
|
35%
|
|
Urothelial Carcinoma
|
86%
|
|
|
Miettinen, et al (clone L50-823, dilution of 1:500; Biocare Medical, Concord, CA)
|
Tumor Type
|
%
|
N
|
|
Adrenocortical Carcinoma
|
11%
|
N=27
|
|
Basal Cell Ca., Skin
|
98%
|
N=62
|
|
Benign Skin Adnexal Tumors
|
100%
|
N=24
|
|
Breast, Ductal Ca., Primary
|
92%
|
N=179
|
|
Breast, Ductal Ca., Metastatic
|
96%
|
N=51
|
|
Breast, Lobular Carcinoma
|
100%
|
N=38
|
|
Malignant Mesothelioma
|
58%
|
N=64
|
|
Germ Cell Tumor, Choriocarcinoma
|
100%
|
N=11
|
|
Germ Cell Tumor, Endodermal Sinus Tumor
|
100%
|
N=6
|
|
Pancreas, Adenocarcinoma
|
37%
|
N=62
|
|
Renal Cell Carcinoma, Chromophobe
|
51%
|
N=35
|
|
Renal Oncocytoma
|
17%
|
N=35
|
|
Salivary Gland, Adenoid Cystic Carcinoma
|
29%
|
N=17
|
|
Salivary Gland, Ductal Carcinoma
|
43%
|
N=14
|
|
SCC – Skin
|
81%
|
N=31
|
|
SCC – Cervix
|
33%
|
N=21
|
|
SCC – Larynx
|
16%
|
N=36
|
|
SCC – Lung
|
12%
|
N=74
|
|
Urothelial Carcinoma – Low Grade
|
100%
|
N=22
|
|
Urothelial Carcinoma – High Grade
|
84%
|
N=32
|
No GATA-3 expression found in the following: Seminoma (n=76), Pure Embryonal Carcinoma (n=5), Lung Small Cell Carcinoma (n=30), Lung Carcinoid (n=11), Small Intestine Carcinoid (n=18), Merkel Cell Carcinoma (n=4), Ovary Non-Serous Carcinomas (n=25), Pancreatic Neuroendocrine Tumor, Rectal Adenocarcinoma (n=27), and Thymoma (n=41). (Miettinen, et al)
GATA-3 expression in 0-5% of tumors was found in the following: Stomach Adenocarcinoma (n=133), Thyroid Papillary and Follicular Carcinomas (n=75), Renal Cell Carcinoma NOT Chromophobe (n=154), Prostate Adenocarcinoma (n=95), Hepatocellular Carcinoma (n=47), and Colon Adenocarcinoma (n=142). (Miettinen, et al)
GATA-3 expression in 6-10% of tumors was found in the following: Anaplastic Thyroid Carcinoma (n=11), Ovarian Serous Carcinoma (n=73), Lung Adenocarcinoma (n=71), Cholangiocarcinoma (n=57), and Endometrial Adenocarcinoma (n=89). (Miettinen, et al)
Liu, et al. (GATA-3 [HG3-31]:sc-268; Santa Cruz Biotech, Santa Cruz, CA)
|
Tumor
|
%
|
N
|
|
Seminoma
|
0%
|
N=30
|
|
Embryonal Carcinoma
|
0%
|
N=24
|
|
Yolk Sac Tumor
|
0%
|
N=12
|
|
Lung Neuroendocrine Carcinoma
|
0%
|
N=61
|
|
Lung Adenocarcinoma
|
0%
|
N=61
|
|
Lung SCC
|
0%
|
N=49
|
|
Papillary Thyroid Carcinoma
|
0%
|
N=47
|
|
Follicular Thyroid Carcinoma
|
0%
|
N=37
|
|
Medullary Thyroid Carcinoma
|
0%
|
N=10
|
|
Clear Cell RCC
|
0%
|
N=82
|
|
Papillary RCC
|
0%
|
N=20
|
|
Colon Adenocarcinoma
|
0%
|
N=43
|
|
Esophageal Adenocarcinoma
|
0%
|
N=30
|
|
Gastric Adenocarcinoma
|
0%
|
N=21
|
|
Pancreatic Adenocarcinoma
|
0%
|
N=50
|
|
Urothelial Carcinoma
|
86%
|
N=72
|
|
Prostatic Adenocarcinoma
|
0%
|
N=136
|
|
Cholangiocarcinoma
|
0%
|
N=11
|
|
Breast Ductal Carcinoma
|
91%
|
N=99
|
|
Breast Lobular Carcinoma
|
100%
|
N=48
|
|
Endocervical Adenocarcinoma
|
0%
|
N=17
|
|
Endometrial Carcinoma
|
2%
|
N=96
|
|
Ovarian Serous Carcinoma
|
0%
|
N=56
|
|
Hepatocellular Carcinoma
|
0%
|
N=18
|
|
Pancreatic Endocrine Neoplasm
|
0%
|
N=15
|
|
Skin Melanoma
|
0%
|
N=100
|
Clark, et al.
|
Tumor
|
%
|
N
|
|
Breast
|
|
|
|
– HR+/Her2=
|
99%
|
N=131
|
|
– HR+/Her2+
|
100%
|
N=18
|
|
– HR=/Her2+
|
100%
|
N=7
|
|
– Triple Negative
|
73%
|
N=30
|
|
Endocervix
|
18%
|
N=34
|
|
Vulva/Cervix SCC
|
60%
|
N=10
|
|
Endometrium
|
7%
|
N=55
|
|
Ovary
|
10%
|
N=50
|
|
Bladder
|
95%
|
N=22
|
|
Liver (cholangiocarcinoma)
|
3%
|
N=62
|
|
Pancreas
|
10%
|
N=30
|
|
Stomach
|
2%
|
N=62
|
Parathyroid vs. Thyroid
GATA-3 has been shown to be expressed in >95% of parathyroid samples (n=25 combined) consisting of hyperplastic glands and adenomas, whereas no thyroid tissues showed GATA-3 expression.
Interestingly, IHC for PTH only stained between 1/3 and 2/3rds of parathyroid samples. Therefore, GATA-3 combined with TTF-1 may be very helpful in differentiating between thyroid and parathyroid lesions in small samples (e.g. thyroid FNAs). (Takada, et al.)
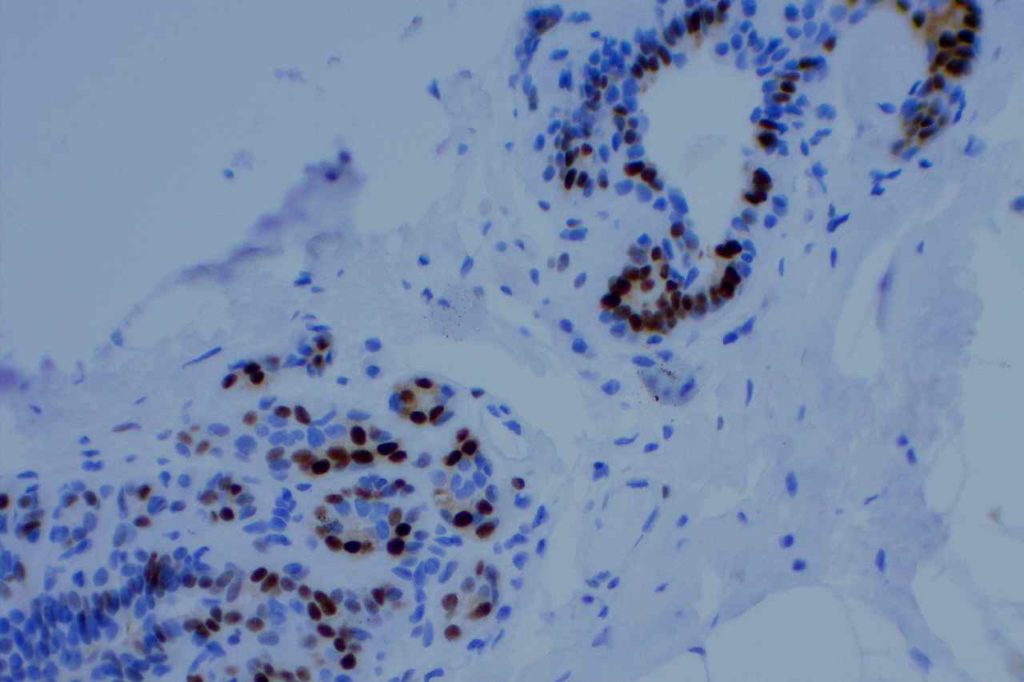
Photomicrographs
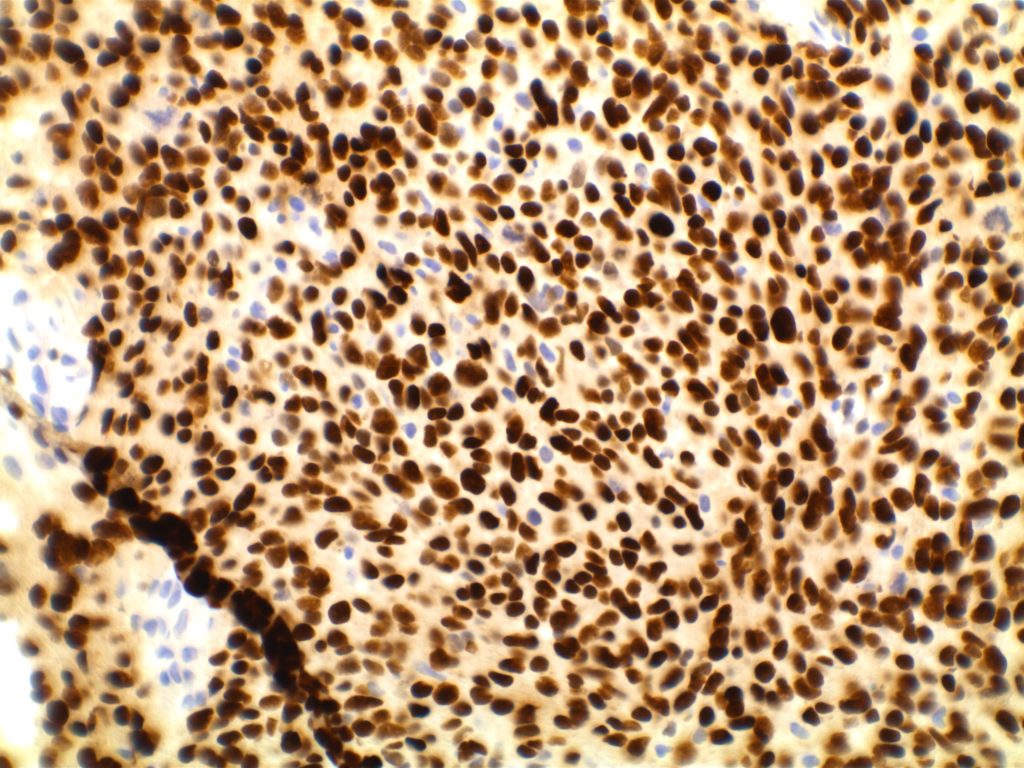
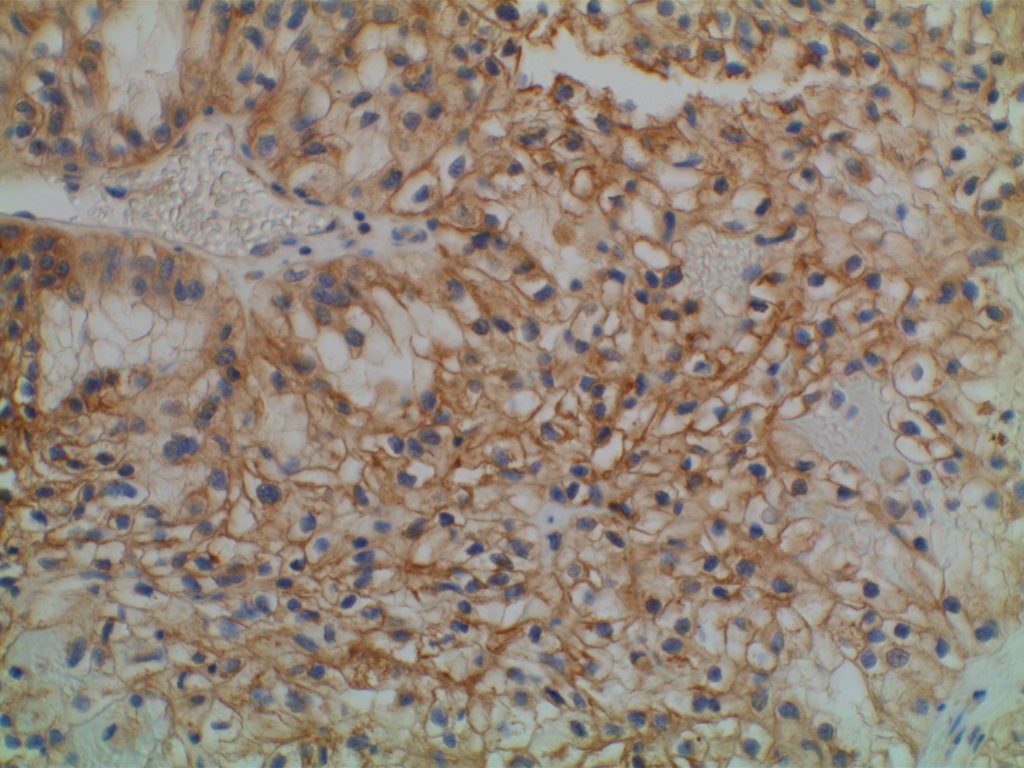
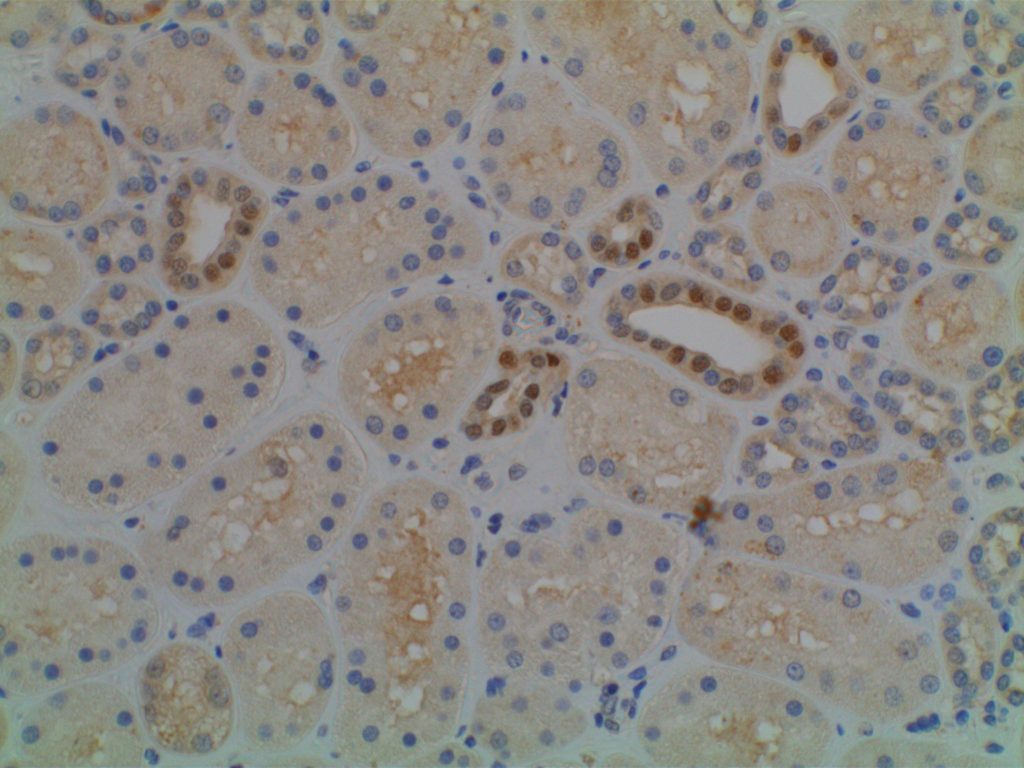
References
Liu, H., Shi, J., Wilkerson, M. L., & Lin, F. (2012). Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. American Journal of Clinical Pathology, 138(1), 57–64. doi:10.1309/AJCP5UAFMSA9ZQBZ
Miettinen, M., McCue, P. A., Sarlomo-Rikala, M., Rys, J., Czapiewski, P., Wazny, K., et al. (2014). GATA3: A Multispecific But Potentially Useful Marker in Surgical Pathology: A Systematic Analysis of 2500 Epithelial and Nonepithelial Tumors. The American Journal of Surgical Pathology, 38(1), 13–22. doi:10.1097/PAS.0b013e3182a0218f
Ellis, C. L., Chang, A. G., Cimino-Mathews, A., Argani, P., Youssef, R. F., Kapur, P., et al. (2013). GATA-3 immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. The American Journal of Surgical Pathology, 37(11), 1756–1760. doi:10.1097/PAS.0b013e31829cdba7
Zhao, L., Antic, T., Witten, D., Paner, G. P., Taxy, J. B., Husain, A., et al. (2013). Is GATA3 expression maintained in regional metastases?: a study of paired primary and metastatic urothelial carcinomas. The American Journal of Surgical Pathology, 37(12), 1876–1881. doi:10.1097/PAS.0b013e31829e2525
Clark, B. Z., Beriwal, S., Dabbs, D. J., & Bhargava, R. (2014). Semiquantitative GATA-3 Immunoreactivity in Breast, Bladder, Gynecologic Tract, and Other Cytokeratin 7-Positive Carcinomas. American Journal of Clinical Pathology, 142(1), 64–71. doi:10.1309/AJCP8H2VBDSCIOBF
McCleskey BC, Penedo TL, Zhang K, Hameed O, Siegal GP, Wei S. GATA3 Expression in Advanced Breast Cancer: Prognostic Value and Organ-Specific Relapse. Am J Clin Pathol. 2015;144: 756–763.
doi:10.1309/AJCP5MMR1FJVVTPK
Takada, N., Hirokawa, M., Suzuki, A., Higuchi, M., Kuma, S., Miyauchi, A. (2016). Diagnostic value of GATA-3 in cytological identification of parathyroid tissues Endocrine Journal 63(7), 621-626. https://dx.doi.org/10.1507/endocrj.ej15-0700