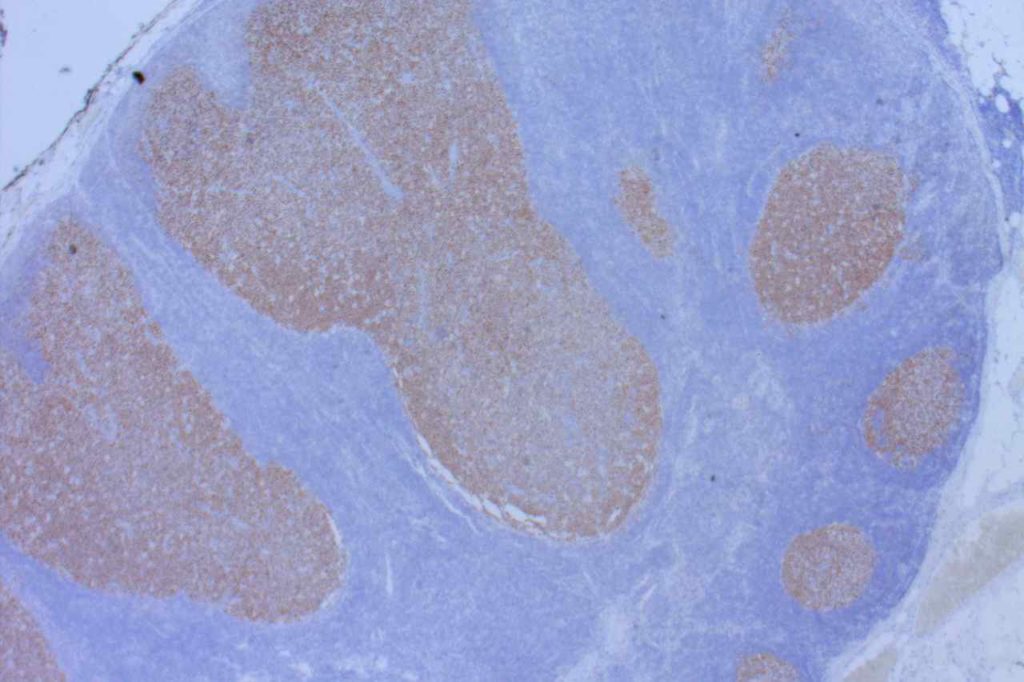
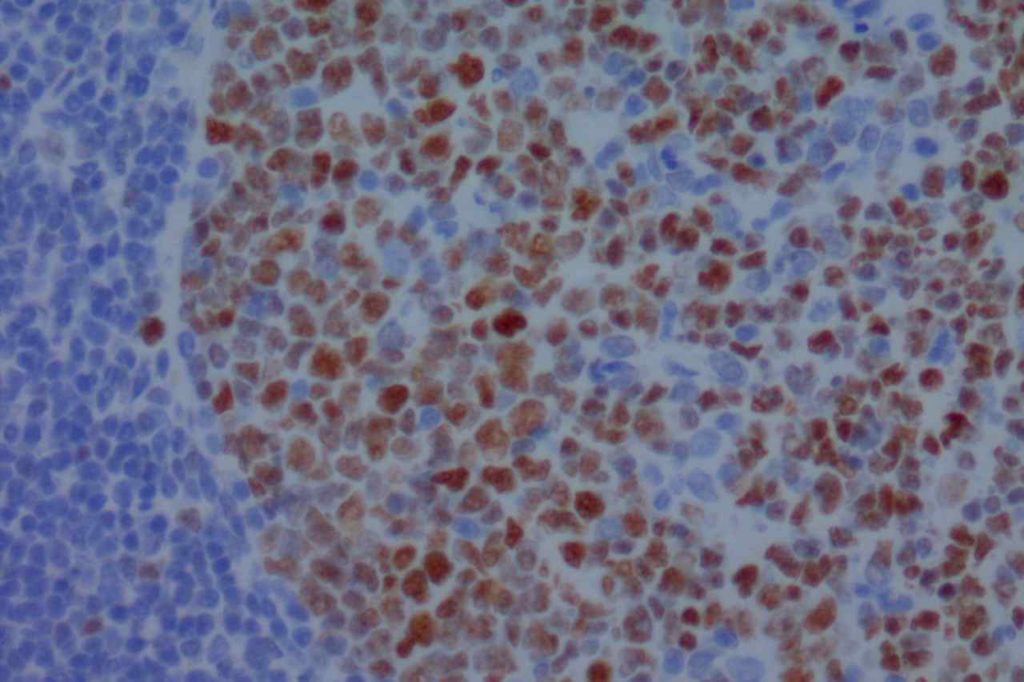
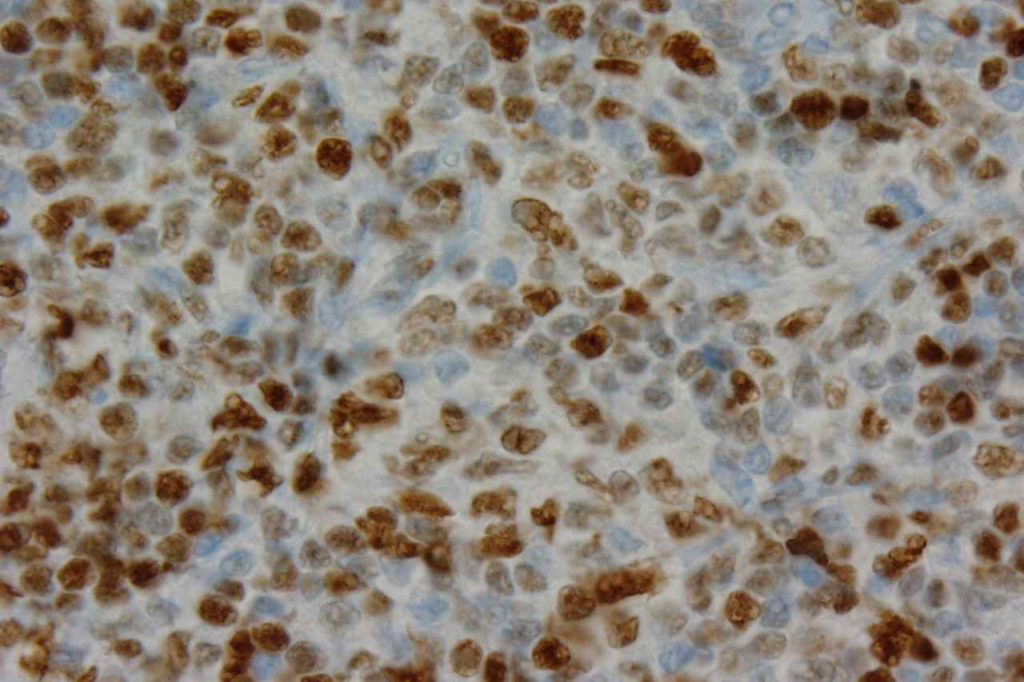
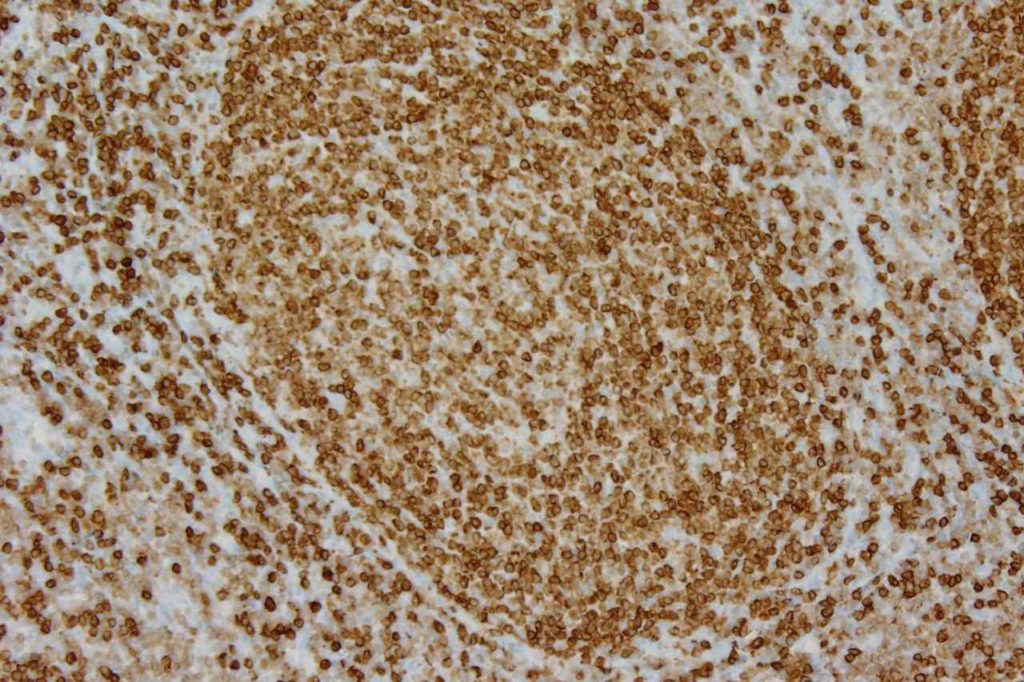
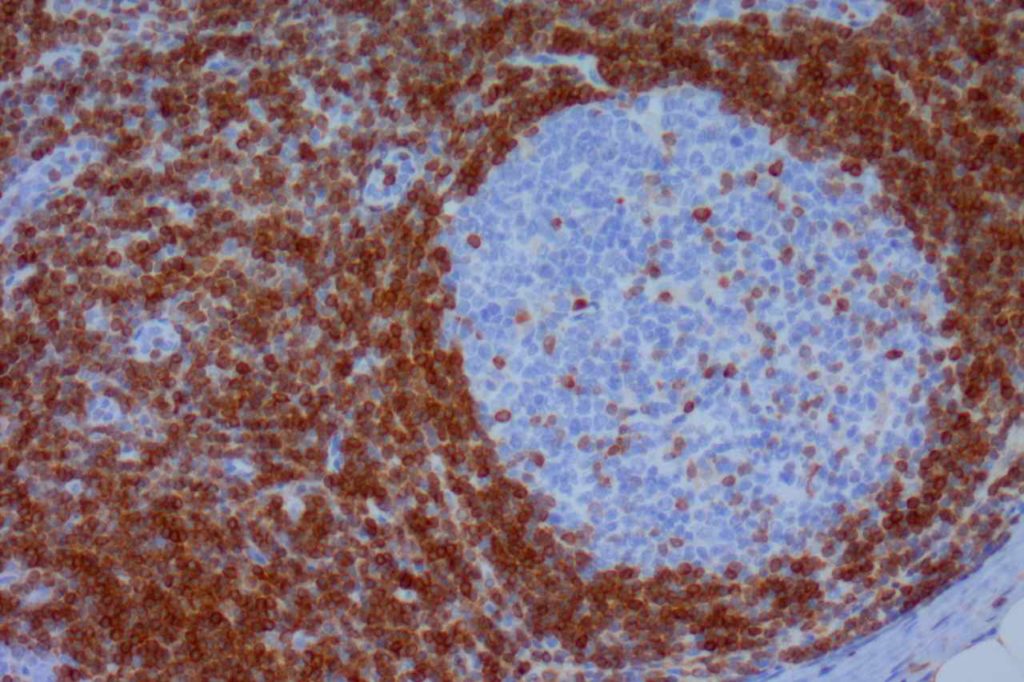
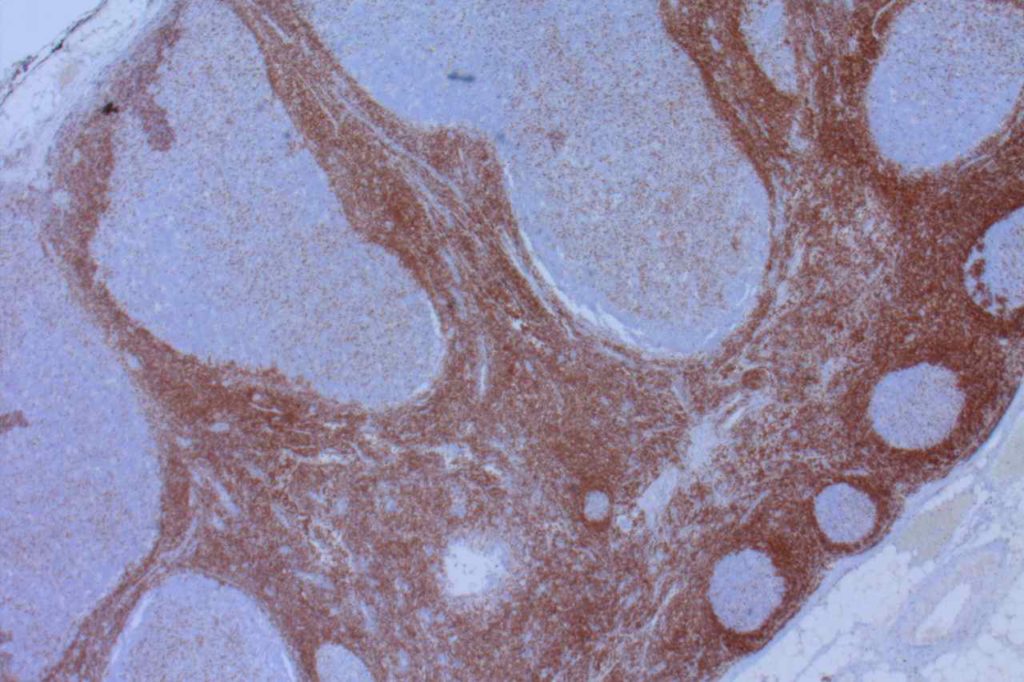
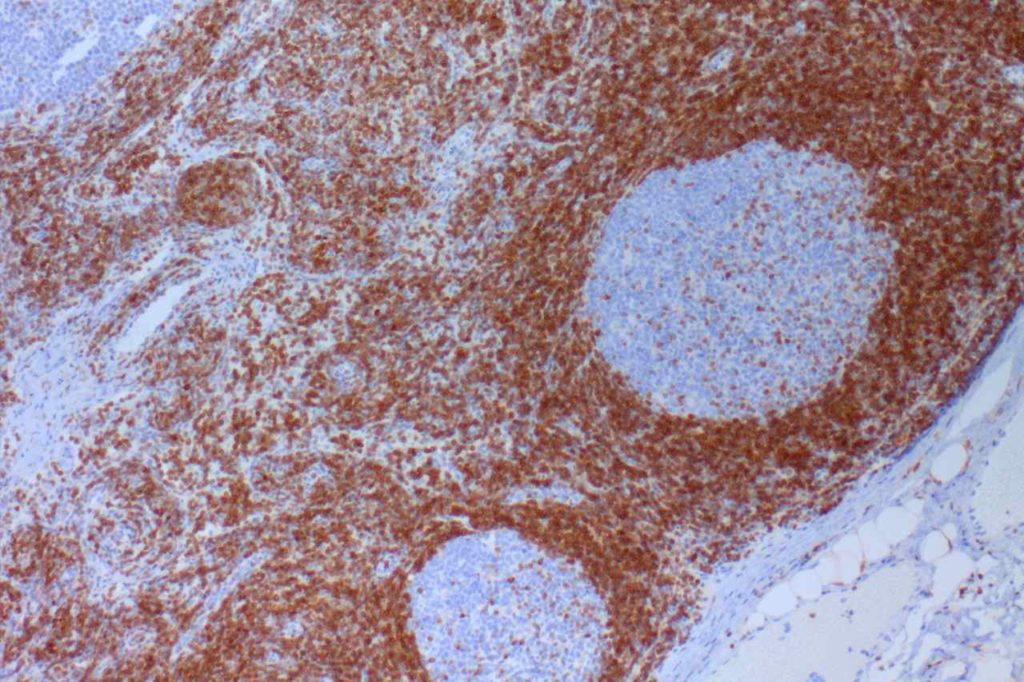
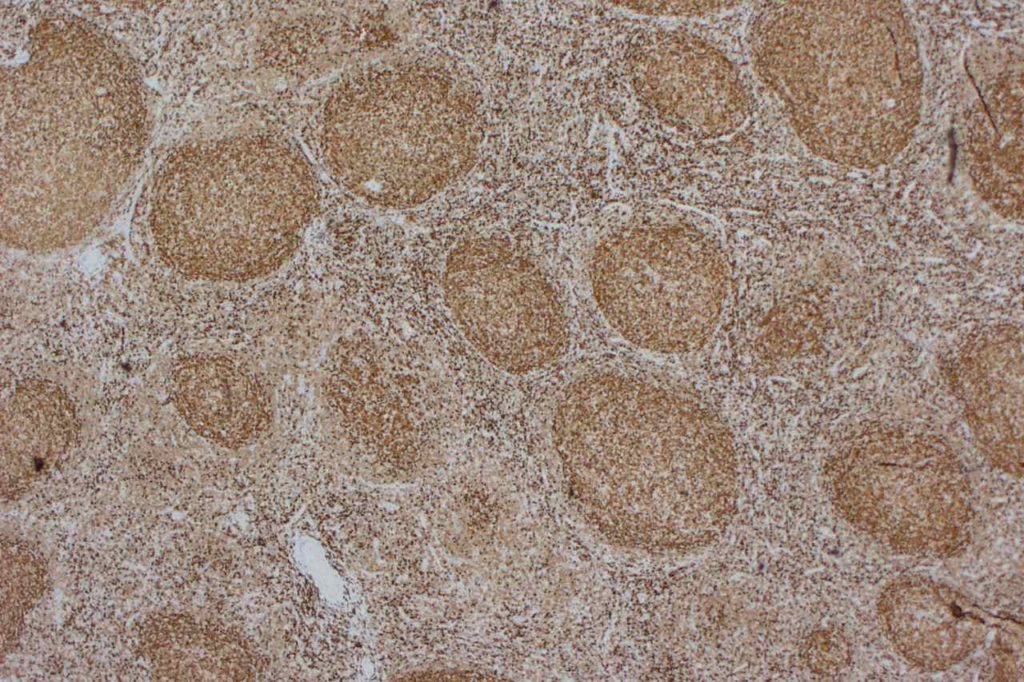
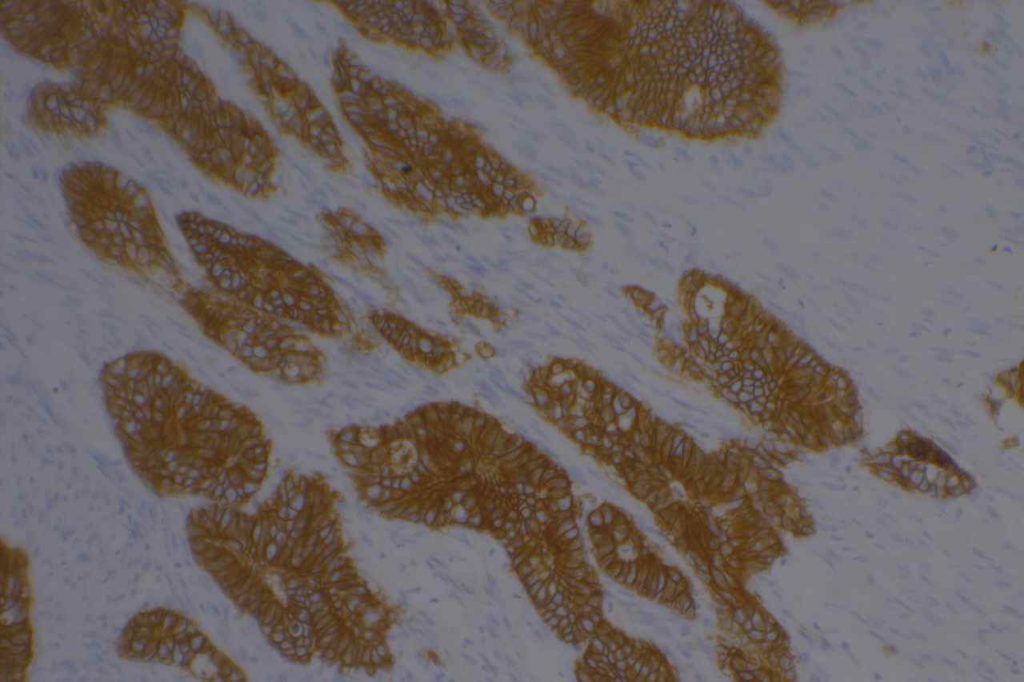
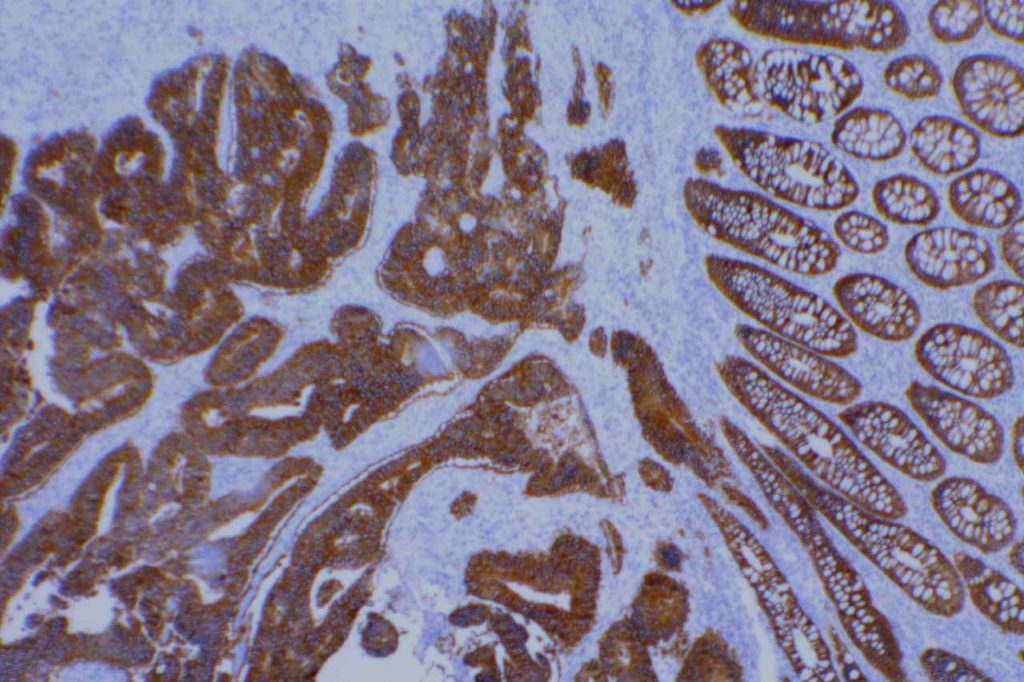
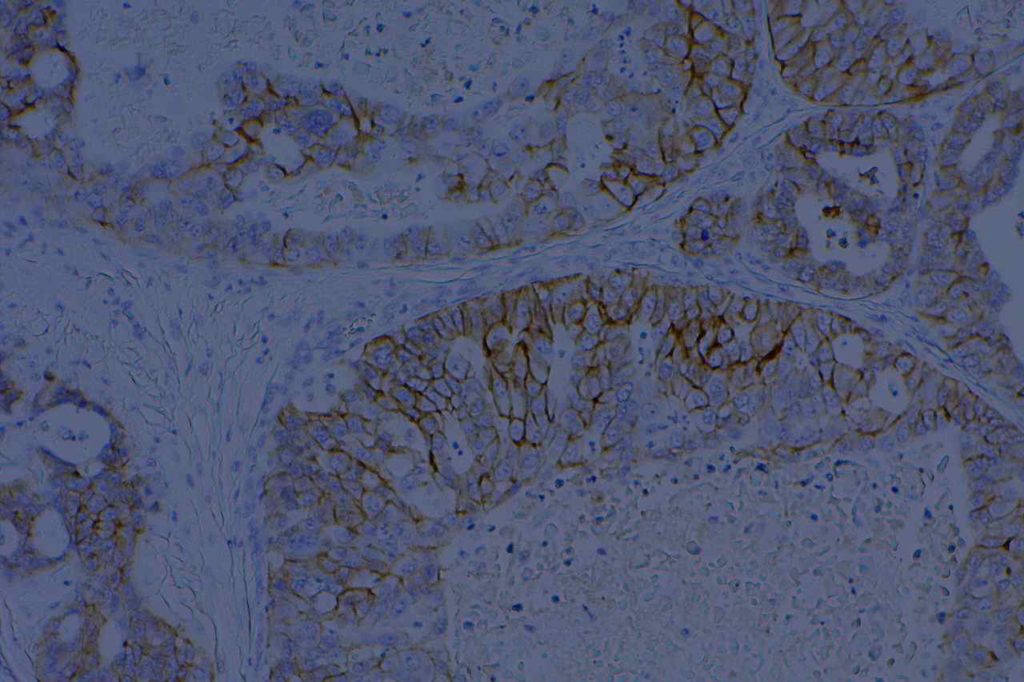
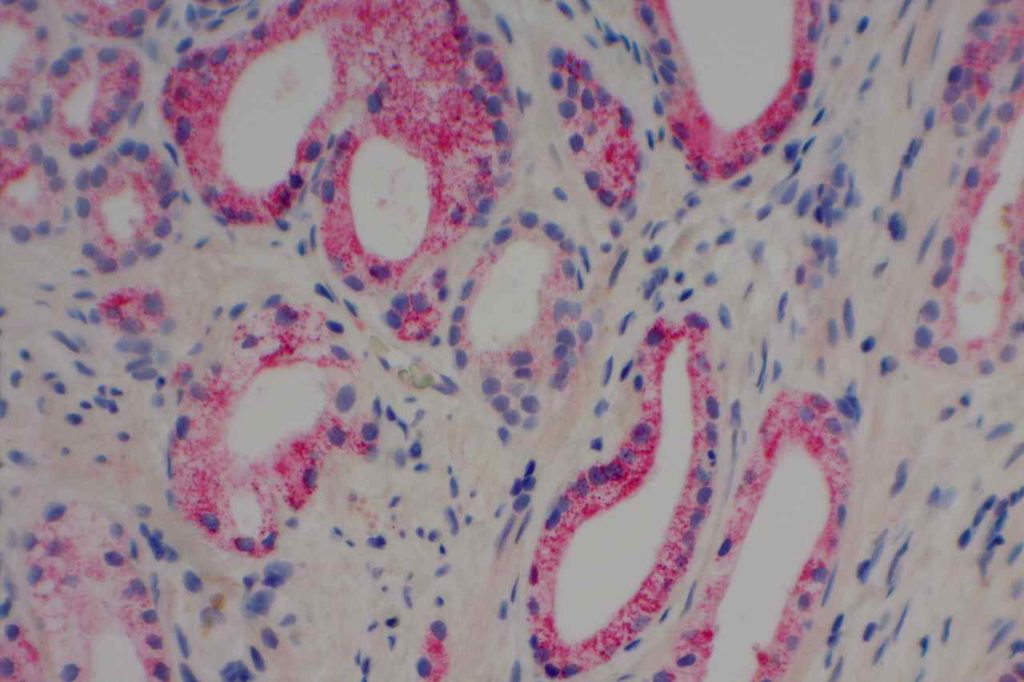
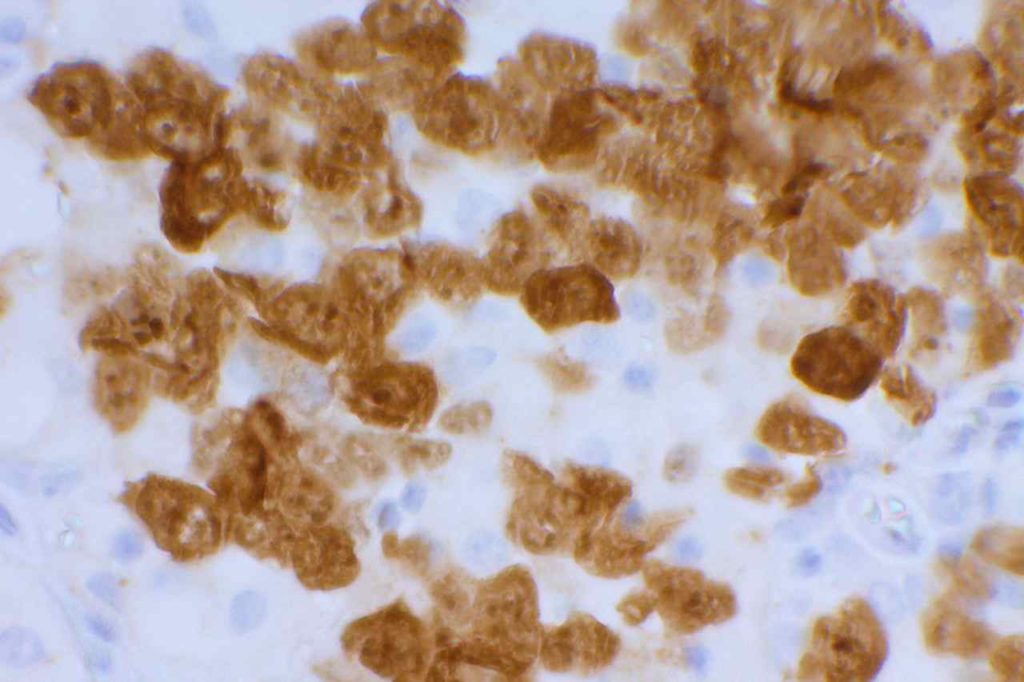
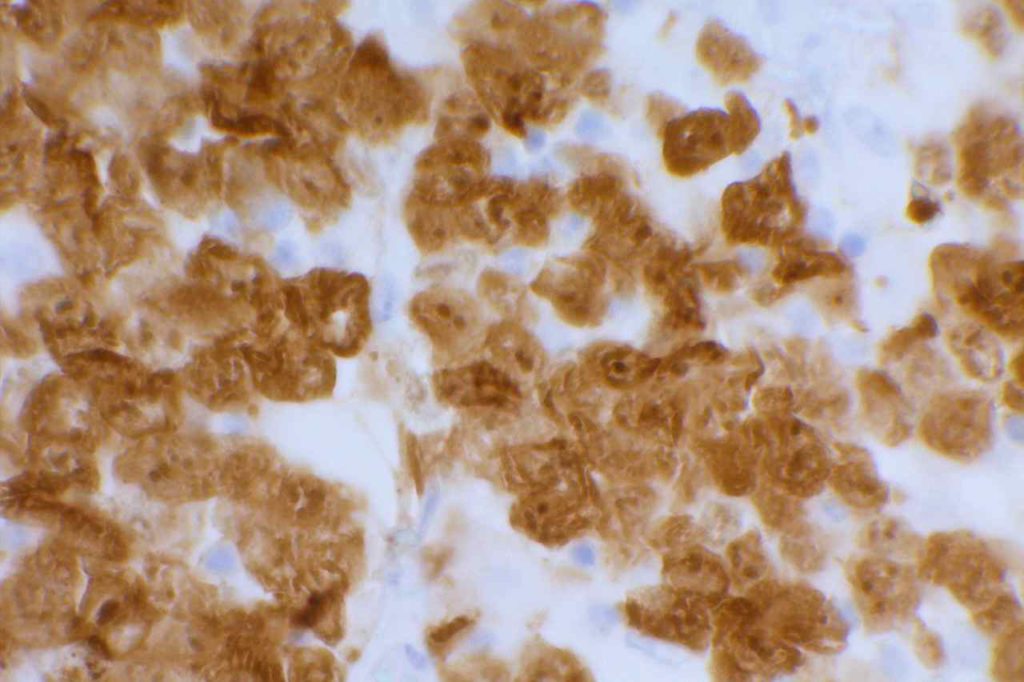
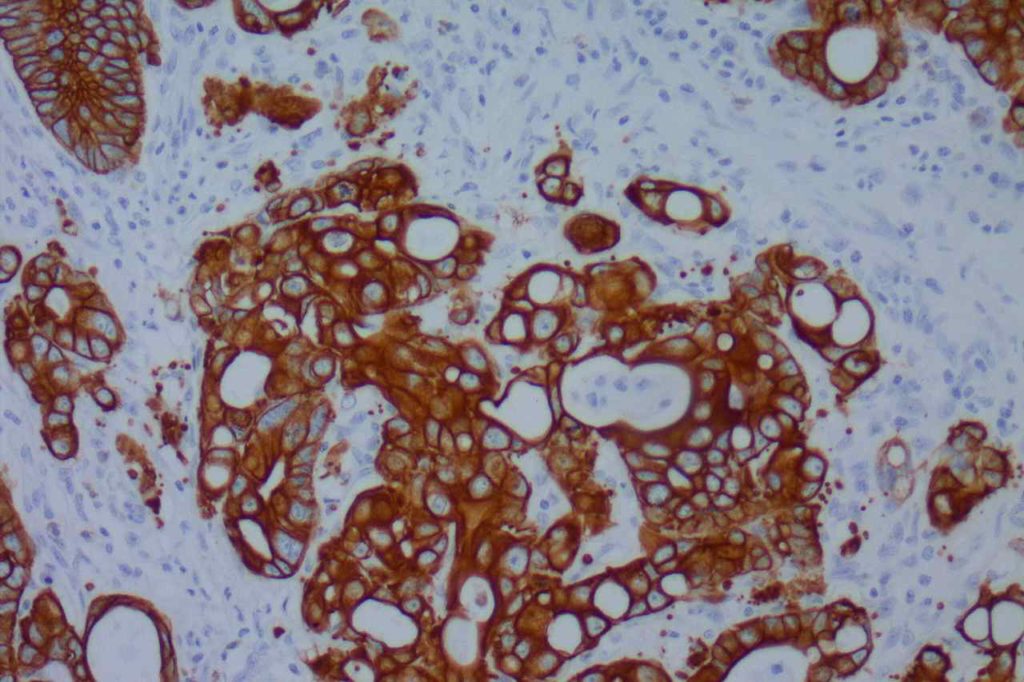
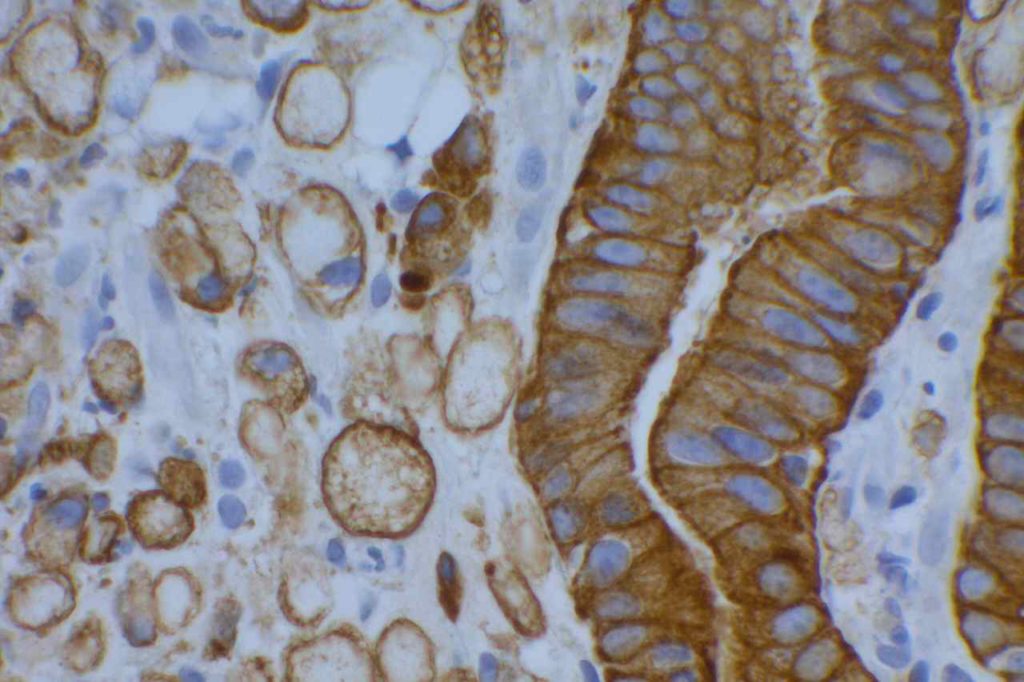
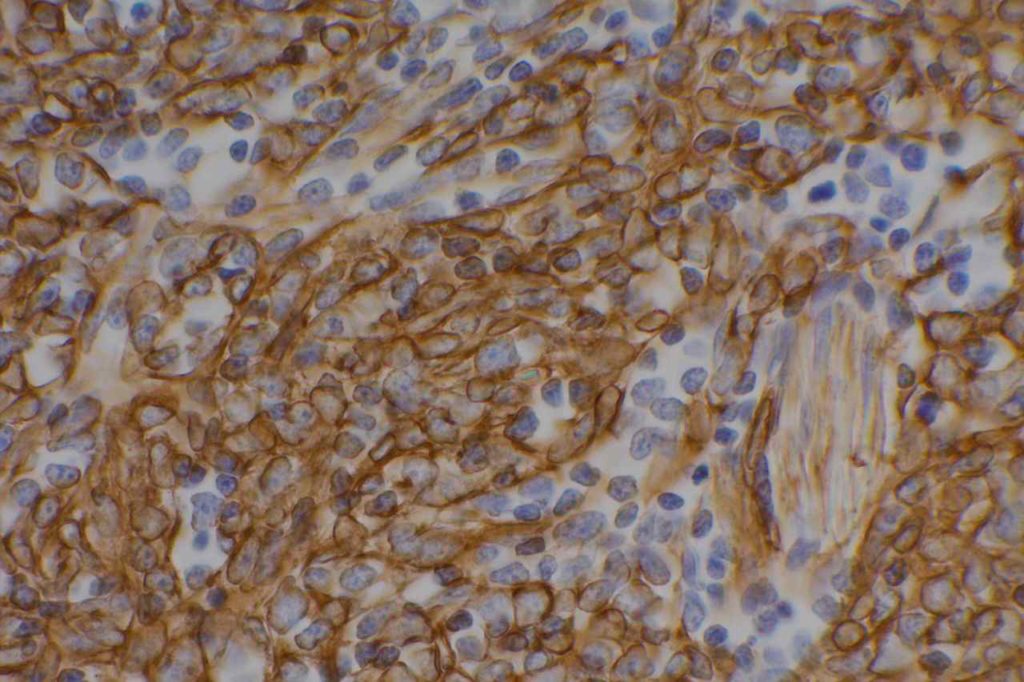
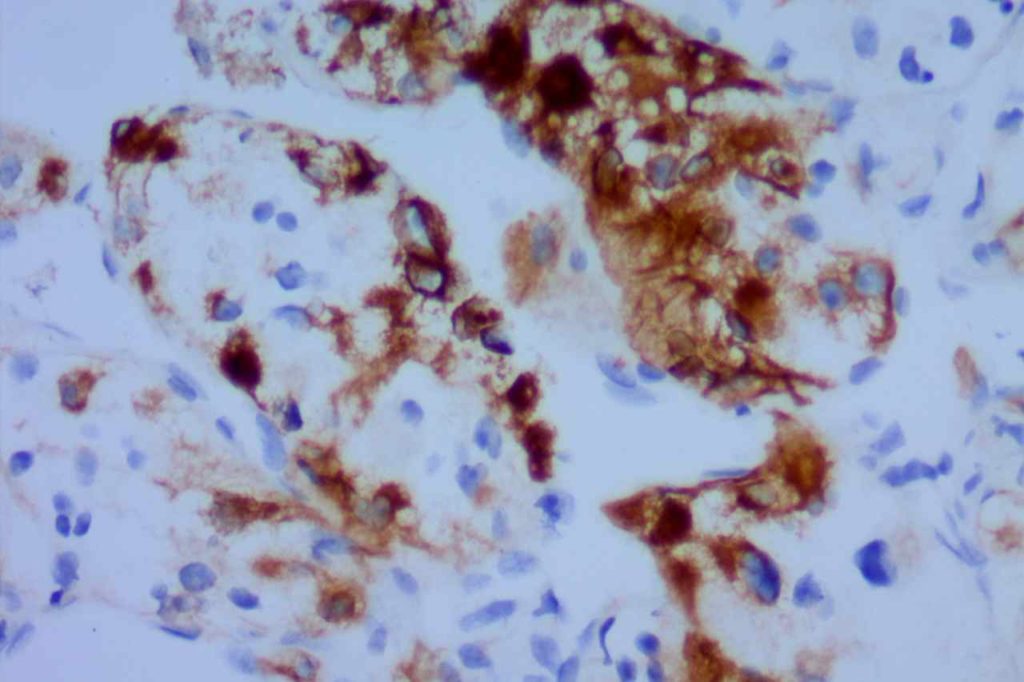
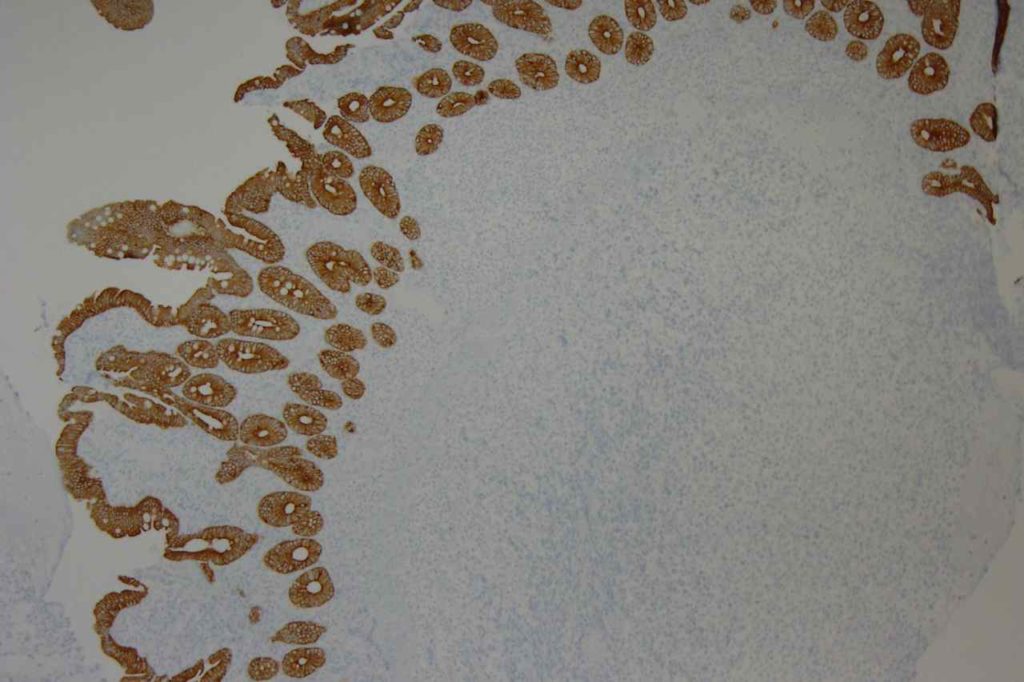
β-catinin is a protein that forms cytoplasmic/membranous complexes with E-cadherin (involved by zonula adherens assembly). It is part of the Wnt signaling pathway, and plays an important role in embryogenesis and neoplasia. The expression may be membraneous, cytoplasmic, or nuclear. The pattern of expression may be important dependent upon what the differential diagnosis one is considering. Expression of beta-catinin is closely regulated by the APC gene. APC mutation associated tumor may have accumulation of protein in the nucleus, and it is the nuclear pattern of expression that is significant in this circumstance. This stain is best used as part of a panel of markers, and it is important to understand the uses and limitations for a given differential diagnosis.
Breast – β-catinin has limited usefulness in stromal proliferations of the breast. While there is documented variability of expression between fibroadenomas (FA) and phyllodes tumors (PT), the specificity of expression pattern limits this stain’s usefulness. (Yang et al.)
Pancreas – nuclear expression of β-catinin is found in pancreatic acing cell carcinoma and solid and pseudo papillary neoplasm, but is negative in pancreatic ductal carcinomas and pancreatic endocrine tumors. Pancreatoblastoma may have focal expression.
Soft Tissue – Cases of fibrzomatosis have been shown to demonstrate nuclear expression of β-catinin (may be focal, e.g. breast biopsies), compared with nodular fascitis, myofibroma, scar, fibrosarcomas, and low-grade fibromyxoid sarcomas. There is still limited data on many entities, which may be in the differential diagnosis of soft tissue lesions. It is important that one understands the usefulness and limitations of this marker.
Thyroid – β catinin shows strong to weak membraneous expression in follicular adenomas and well-differentiated carcinomas. There is nuclear and cytoplasmic expression in poorly differentiated and anapestic carcinomas, and loss of membraneous staining (adverse prognostic indicator).
Uterus – nuclear β-catinin expression may be seen in approximately 50% of low-grade endometrial stromal sarcomas, but not in smooth muscle tumors. About half of high grade endometriod carcinomas will express nuclear beta-catinin, but not in high grade serous carcinomas.
References:
Yang X, Kandil D, Cosar EF, Khan A. Fibroepithelial tumors of the breast: pathologic and immunohistochemical features and molecular mechanisms. Arch Pathol Lab Med. 2014;138: 25–36. doi:10.5858/arpa.2012-0443-RA
Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008;132: 359–372.
Folpe AL, Montgomery EA. The Diagnostic Value of Beta-Catinin Immunihistochemistry. Adv Anat Pathol. 2012;12. doi:10.1186/s13613-016-0144-6