Hematopathology
CD10 (a.k.a. CALLA, common acute lymphoblastic leukemia antigen) is a useful marker for cells of germinal center cell origin and is expressed during the lymphoblastic phase of development. Therefore, this marker is diagnostically helpful in several areas in hematopathology: acute lymphoblastic leukemia (ALL), follicular lymphoma, diffuse large B-cell lymphoma (DLBCL), and Burkitt lymphoma.
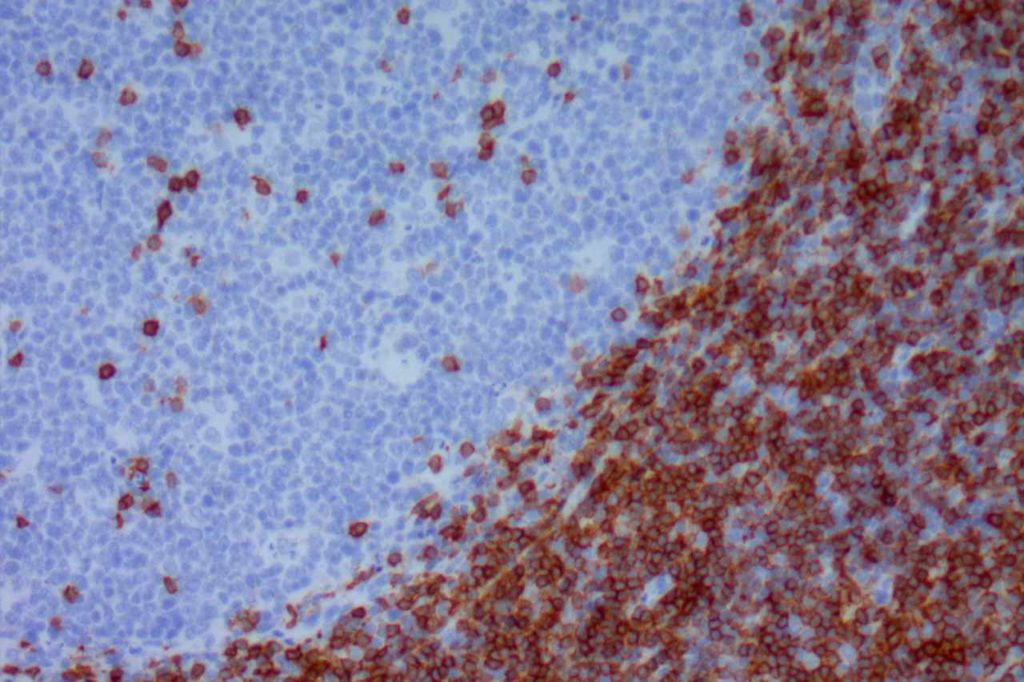
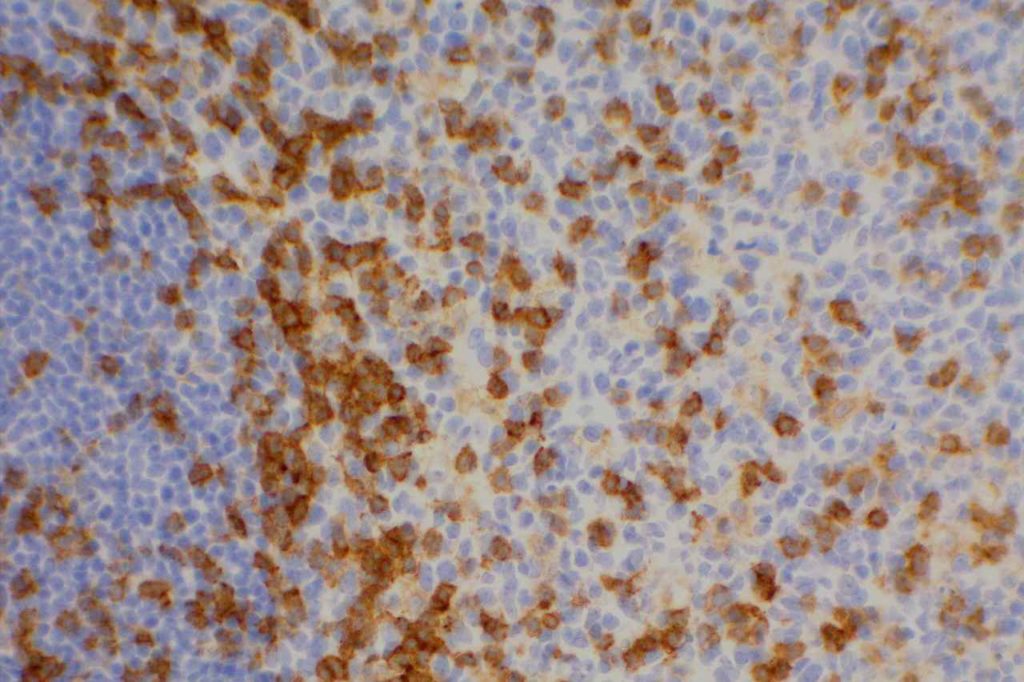
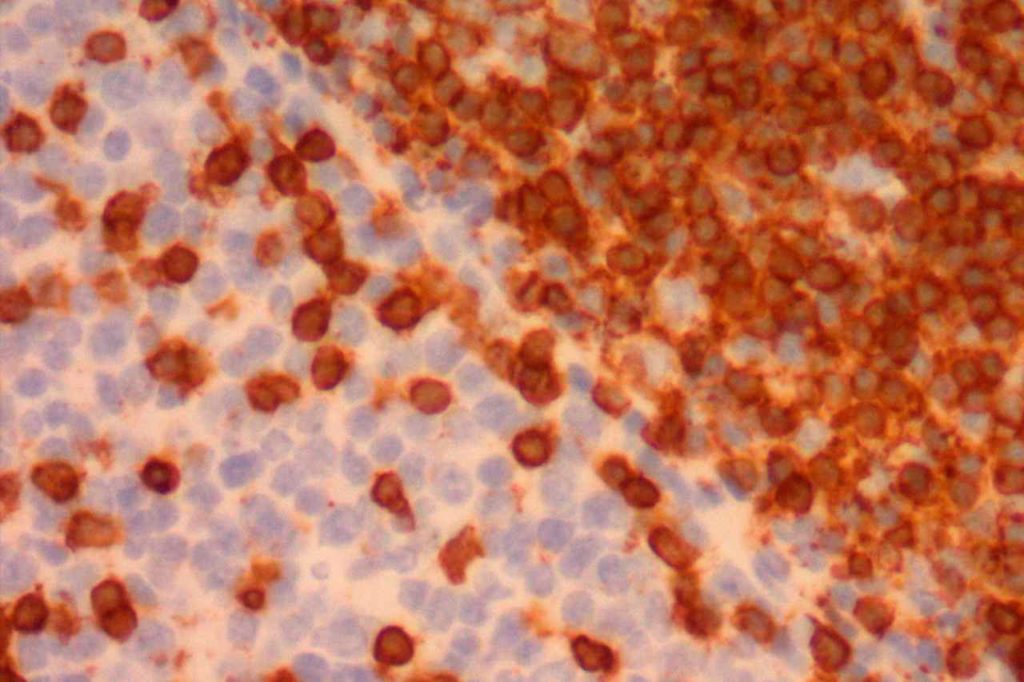
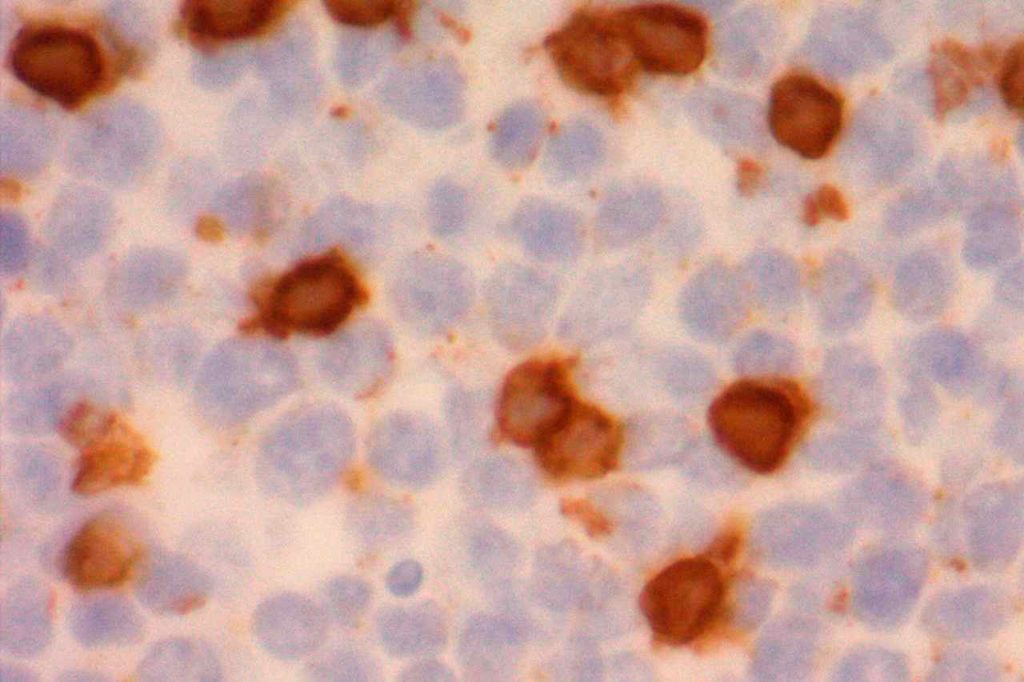
ALL will often show expression of CD10. In fact, CD10 co-expression with TdT is characteristic of ALL (additional expression of T- or B-cell markers will help further classify).
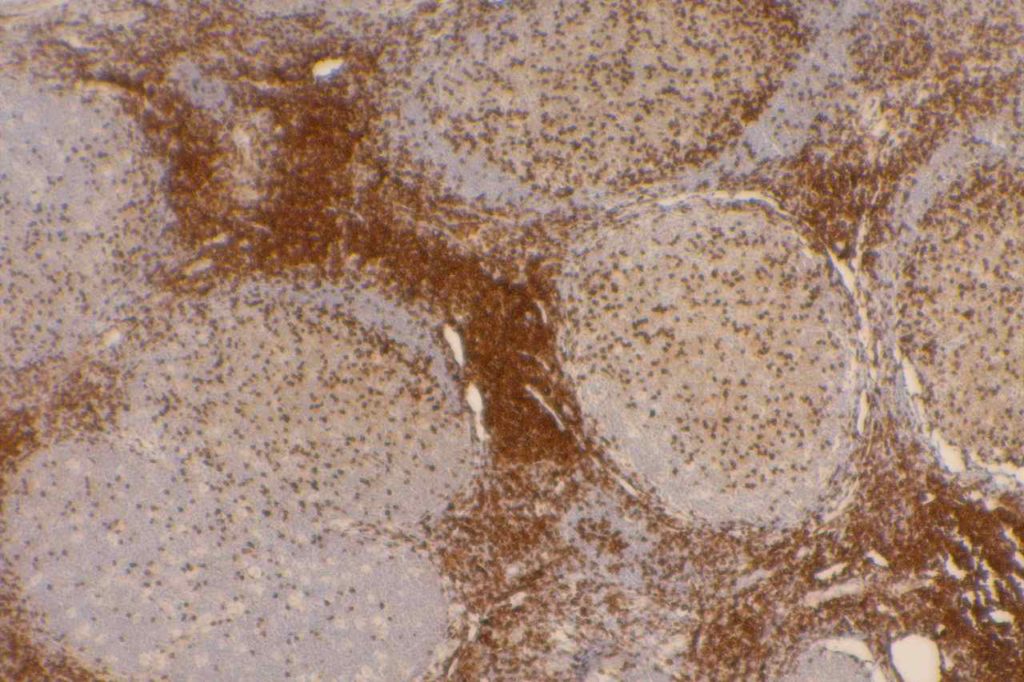
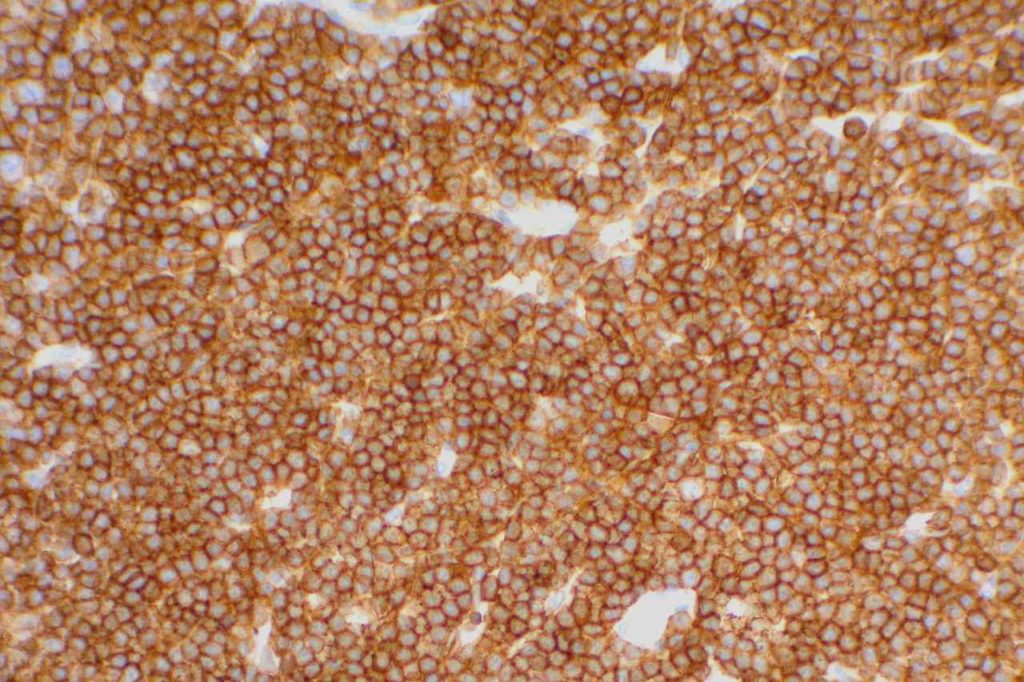
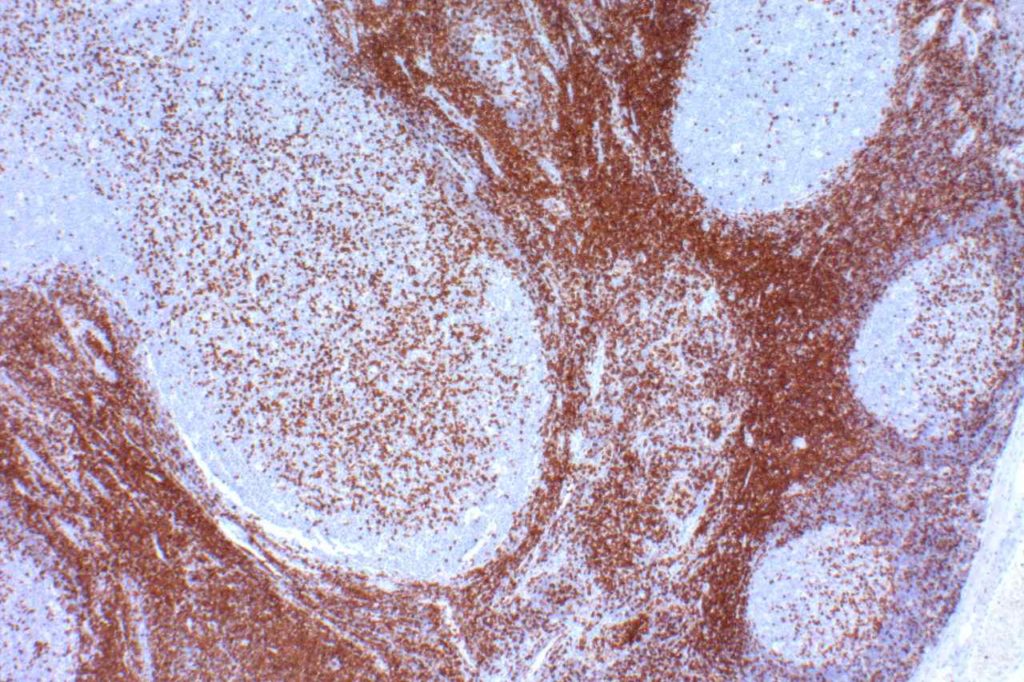
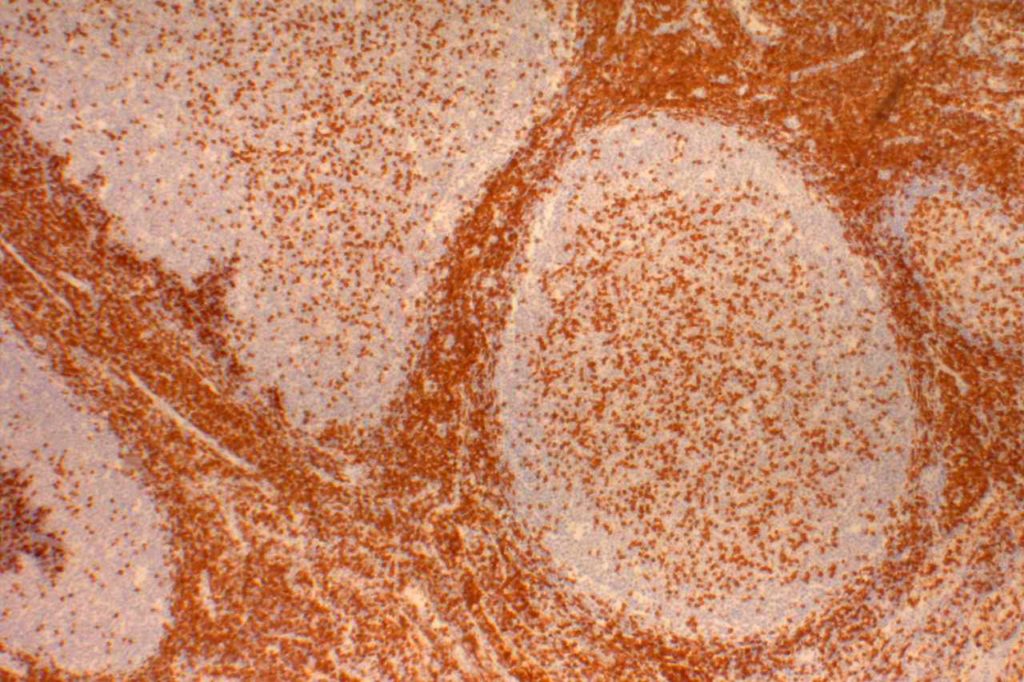
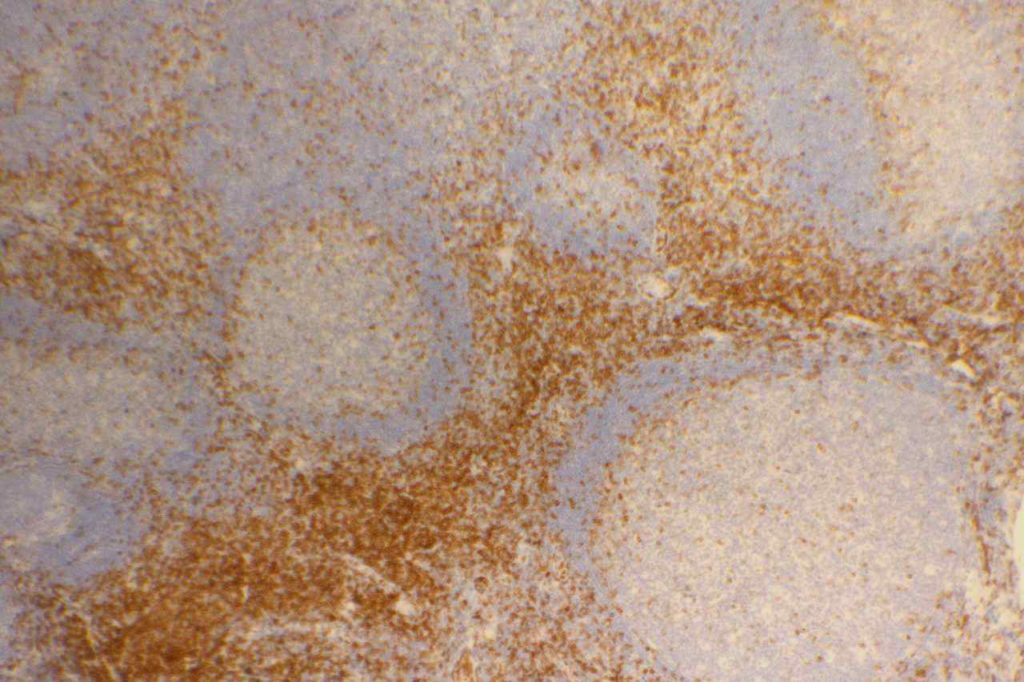
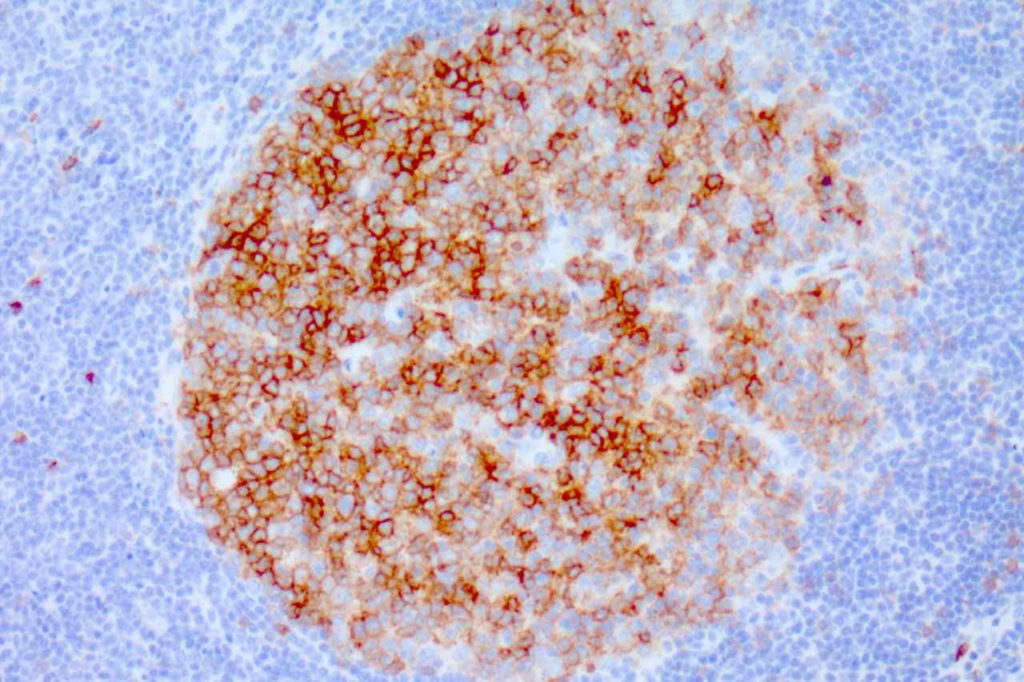
CD10 is a marker of follicle center cell origin, which is characteristic of certain lymphomas including: follicular lymphoma, Burkitt lymphoma, and a subset of DLBCLs.
CD10 can be used as part of a prognostic panel (CD10, bcl-6, and MUM-1) in DLBCL to help separate cases into germinal center and non-germinal center subtypes. The Hans’ (classifier) algorithm method is the most popular, probably due to the simplicity of the algorithm and utilization of IHC markers already present in most laboratories.
Non-Hematopathology
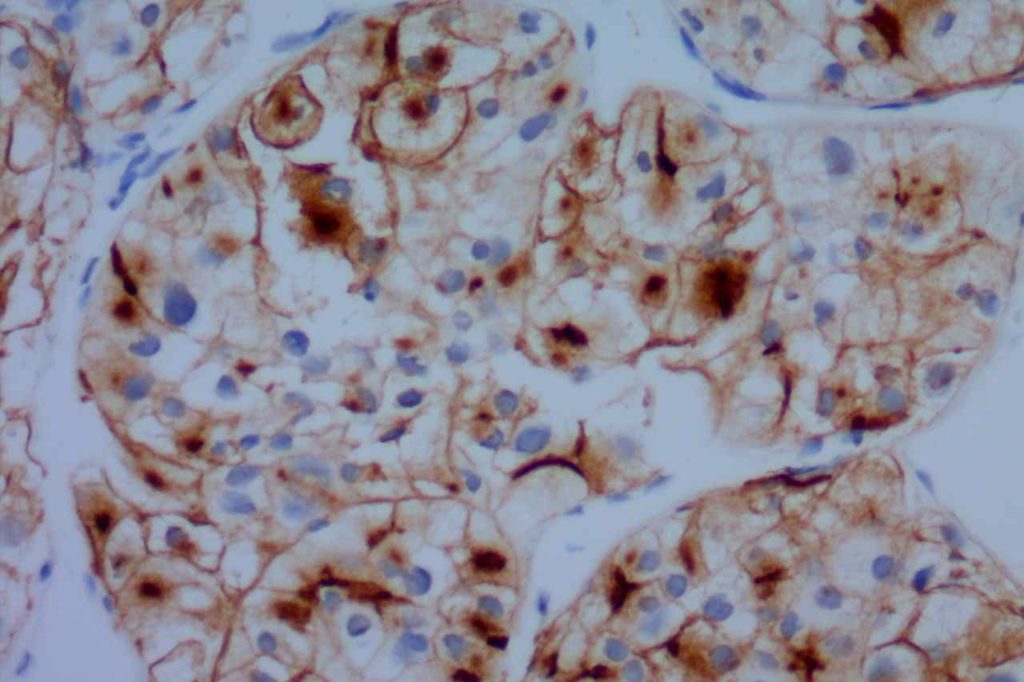
CD10 is a useful marker in non-lymphoid malignancies: renal cell carcinoma and hepatocellular carcinoma. CD10 will have a “bile canaliculi” pattern in HCC. CD10 will also stain endometrial stromal sarcoma, and the “brush boarder” in GI tumors.
Pitfalls
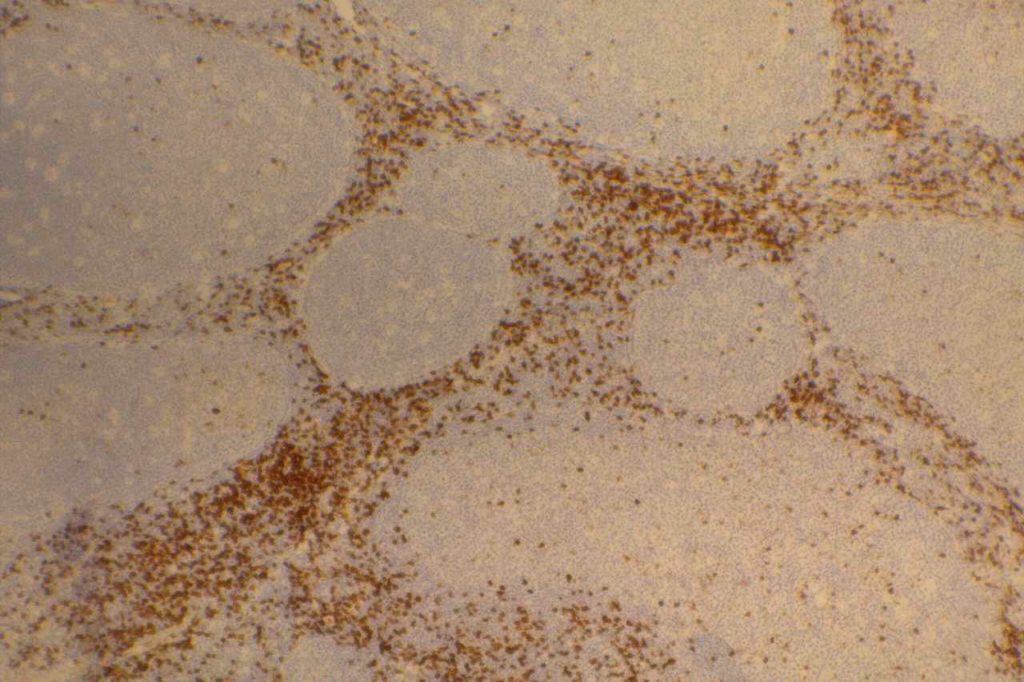
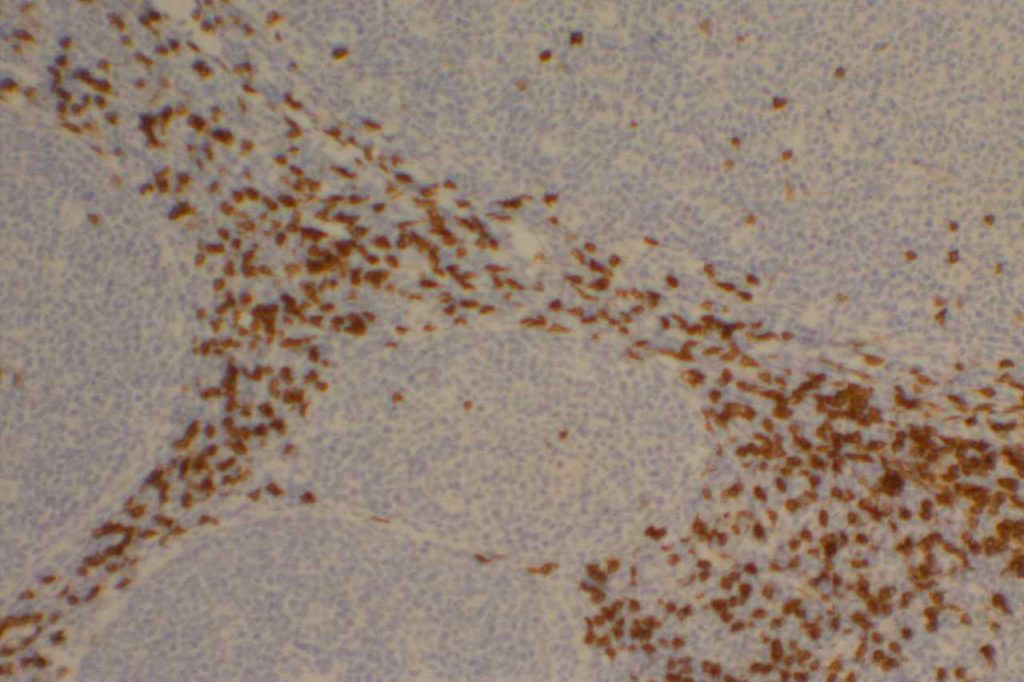
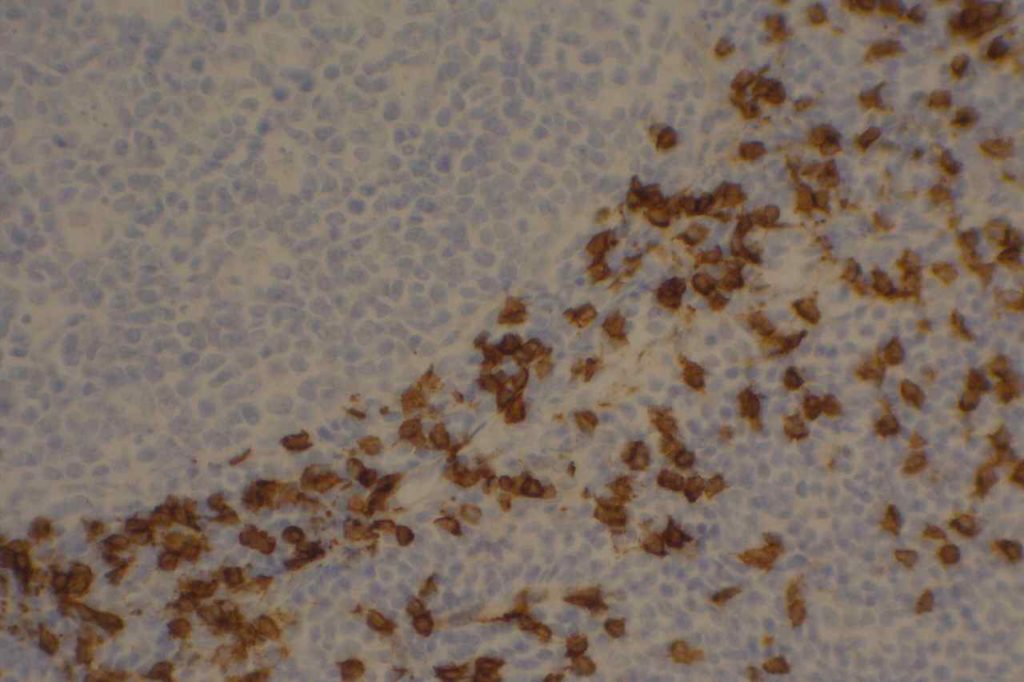
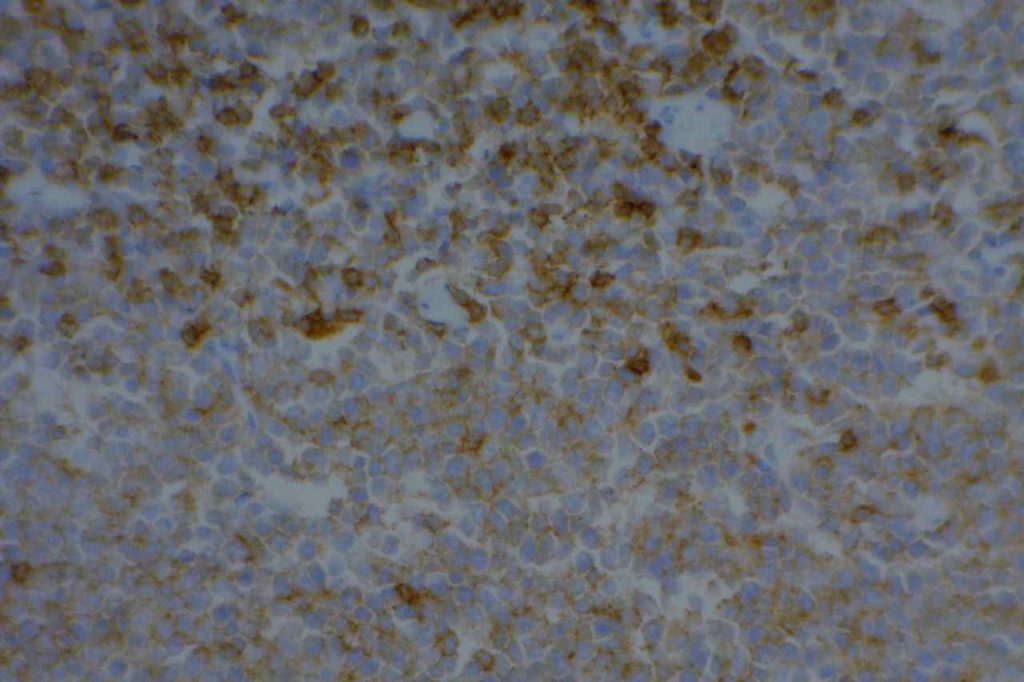
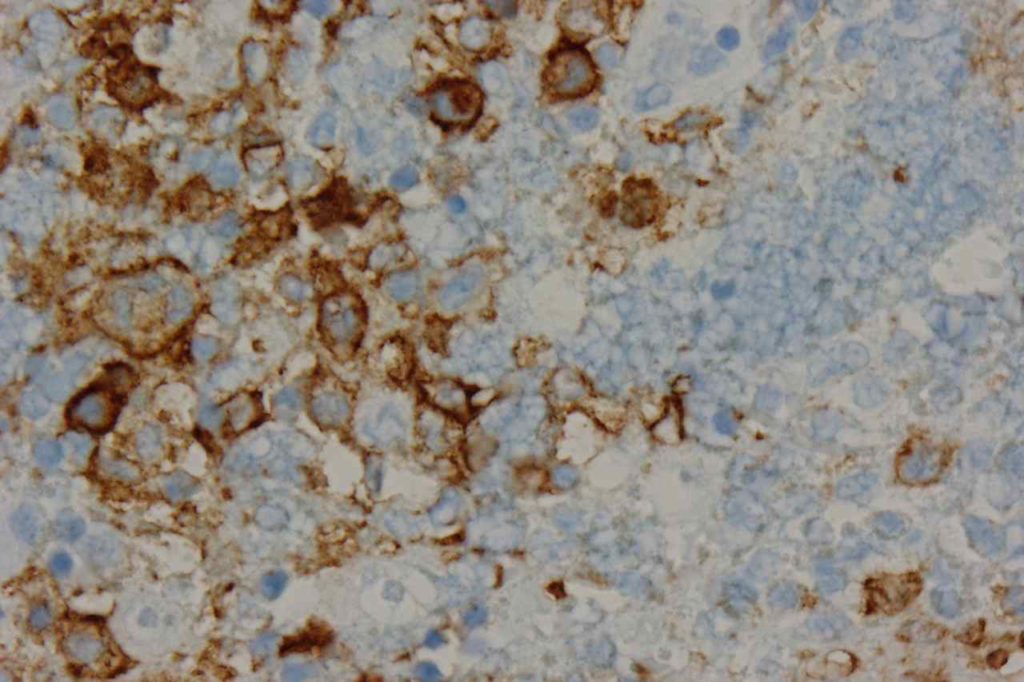
CD10 can appear to have a lot of “non-specific” staining because of staining of dendritic stomal cells. This can cause a pattern similar to reticular fibers, and many describe this as a “reticular pattern,” but the staining does not directly correlate with reticulin staining. Caution should be exercised in using this stain in isolation given its lack of specificity (see below).
CD10 Expression in tumors often studied by CD10 IHC staining
- Acute Lymphoblastic Lymphoma (ALL)
- Normal Lymphoid Precursors (hematogones)
- Bone Marrow: Mature neutrophils (~25%)
- Dendritic Stromal Cells
- Follicular Lymphoma
- Diffuse Large B-cell Lymphoma (DLBCL), 30-60%
- Angioimmunoblastic T-cell Lymphoma
- Burkitt Lymphoma
- Renal Cell Carcinoma (clear cell, papillary and Xp11.2 translocation tumors)
- Endometrial stomal sarcoma
Other tumors/tissues with CD10 expression (20-100% expression)
- Hepatocellular Carcinoma
- Breast myoepithelial cells and stromal fibroblasts
- Cutaneous adnexal neoplasms
- Mesothelioma
- Epithelioid hemangioendotheliomas
- Ovarian carcinoma
- Urothelial carcinoma
- Prostatic adenocarcinoma
- Colon adenocarcinoma
- Melanoma
- Spindle cell carcinoma
- Lung carcinomas
- Pancreatic solid pseudo papillary carcinoma
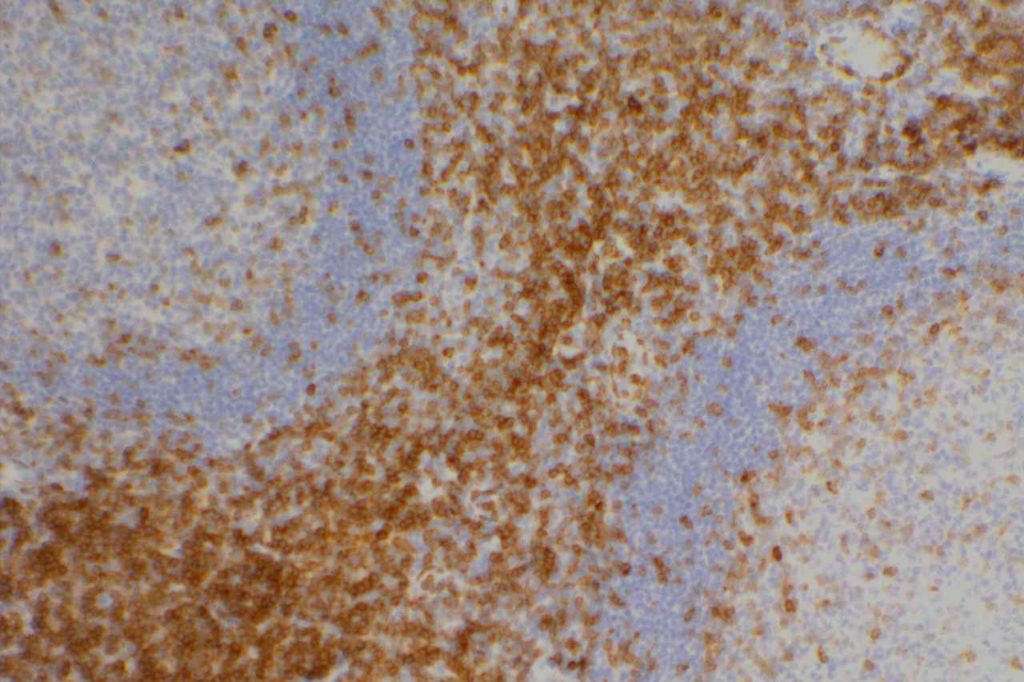
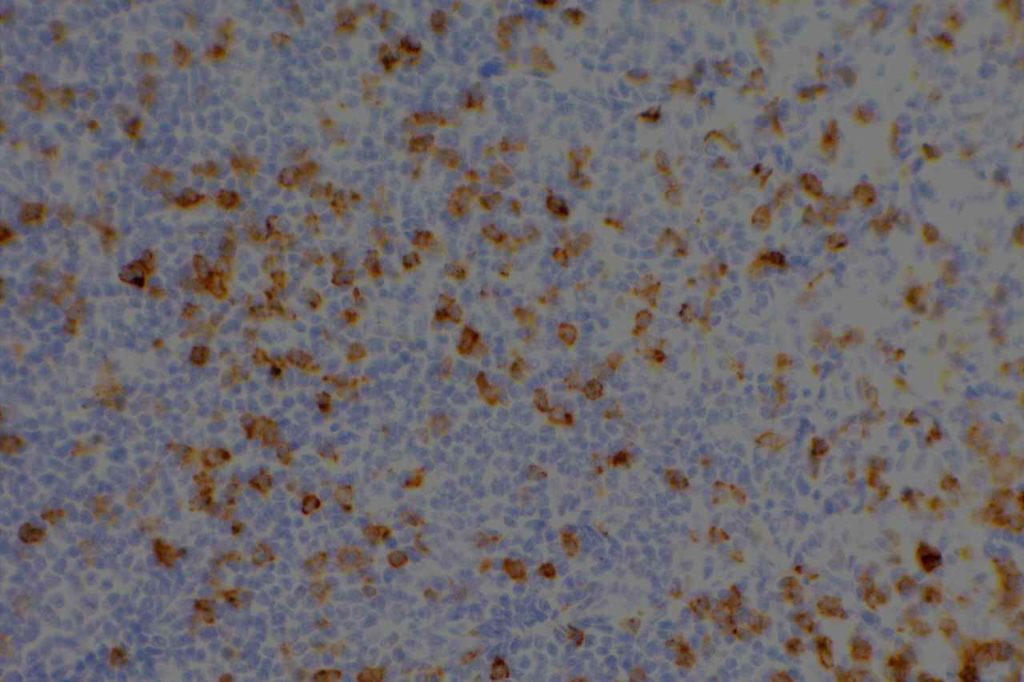
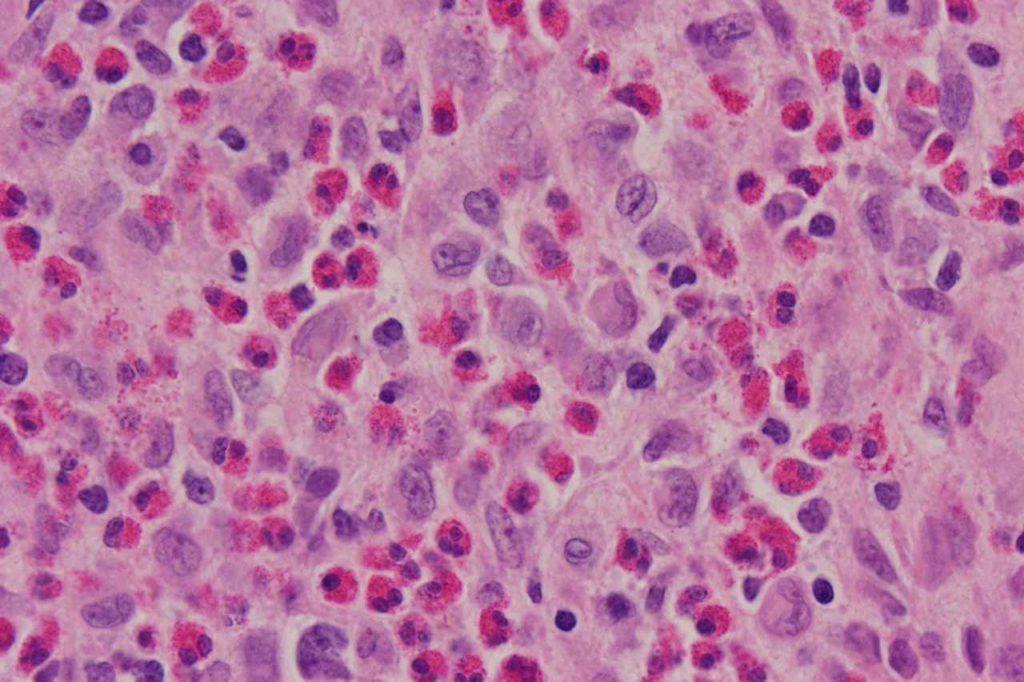
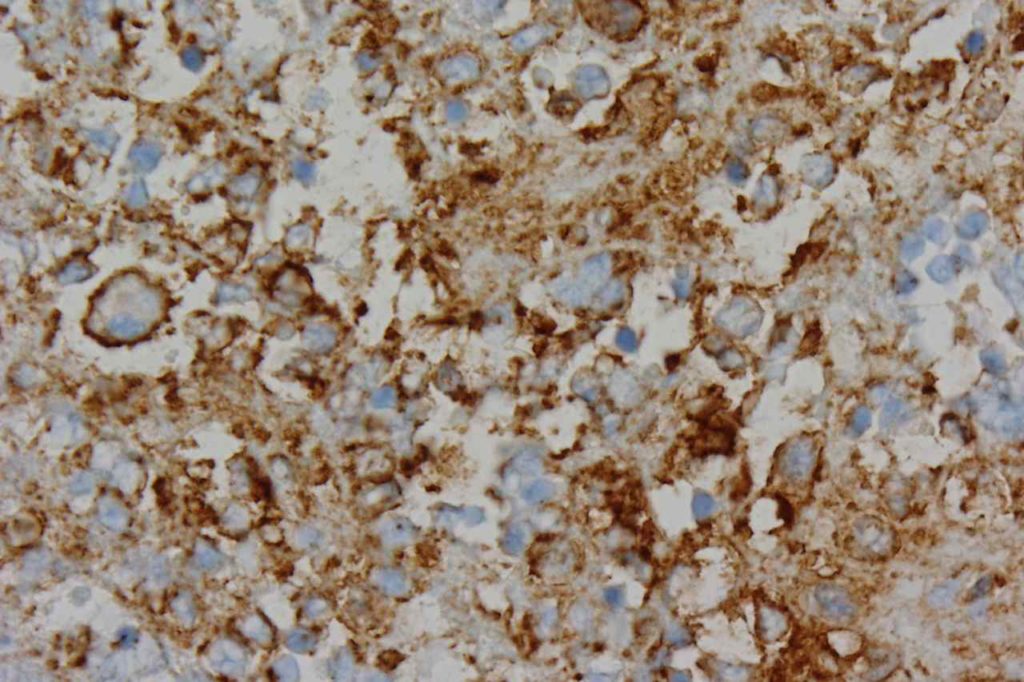
Photomicrographs
References
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103: 275–282. doi:10.1182/blood-2003-05-1545
Tan, P.-H., Cheng, L., Rioux-Leclercq, N., Merino, M. J., Netto, G., Reuter, V. E., et al. (2013). Renal tumors: diagnostic and prognostic biomarkers. (Vol. 37, pp. 1518–1531). Presented at the The American journal of surgical pathology. doi:10.1097/PAS.0b013e318299f12e
Chang, C.-C., McClintock, S., Cleveland, R. P., Trzpuc, T., Vesole, D. H., Logan, B., et al. (2004). Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. The American Journal of Surgical Pathology, 28(4), 464–470.
Tan P-H, Cheng L, Rioux-Leclercq N, Merino MJ, Netto G, Reuter VE, et al. Renal tumors: diagnostic and prognostic biomarkers. 2013. pp. 1518–1531. doi:10.1097/PAS.0b013e318299f12e
Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2010;135: 92–109. Available: http://www.archivesofpathology.org/doi/pdf/10.1043/2010-0478-RAR.1
Dewar R, Fadare O, Gilmore H, Gown AM. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135: 422–429. doi:10.1043/2010-0336-CP.1
Alizadeh AA, Elsen MB, Davis RE, Ma C. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127: 2375–2390. doi:10.1182/blood-2016-01-643569
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 38.