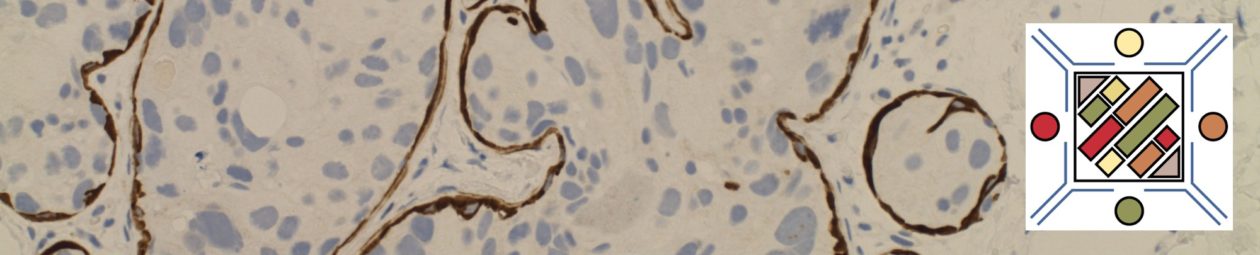
The use of immunohistochemistry (IHC) in many of the MDS syndromes is limited. Identifying an increased blast population is one of the most useful, and may indicate a more aggressive course or transformation to acute myelogenous leukemia (AML). Helpful IHC markers may include:
|
Stain
|
Comment
|
|
CD34 marks immature cells including myeloblasts. In the setting of AML, it is ~70% sensitive. A subset of lymphoblasts may express CD34.
|
|
|
CD117 is a specific myeloid marker, and marks a subset of myeloblasts. The expression is dim, and one often must look at 20-40X to clearly see expression. Mast cells (fried egg looking cell) will have very strong expression.
|
|
|
CD71 marks nucleated erythroid cells. This may be helpful in quantitating and differentiating erythroid cells from myeloid cells. This marker may be set-up as a double stain with CD34.
|
|
|
In the setting of hematopoietic cells, E-Cadherin marks immature erythroid cells. Like CD71, E-Cadherin may be useful to differentiate immature erythroid cells from immature myeloid cells.
|
|
|
TdT is a sensitive lymphoblast (~95%) marker. It is not entirely specific for lymphoblasts, but other markers can help clarify diagnostic difficulties (B and T-cell markers).
|
References
Hematopathology. [edited by] Jaffe, ES. 1st. ed. Elsevier, Inc. © 2011.