Neoplastic
Non-Neoplastic
- Lymph Nodes
- Extra-Nodal
- Bone Marrow
- Peripheral Blood
- Spleen
Collagenous Spherulosis is an uncommon incidental benign finding, which can be confused with other pathologies such as DCIS, carcinoma, and adenoid cystic carcinoma of the breast.
The lesions are characterized by central mucin with epithelial cells (luminal and myoepithelial) radially arranged at the periphery.
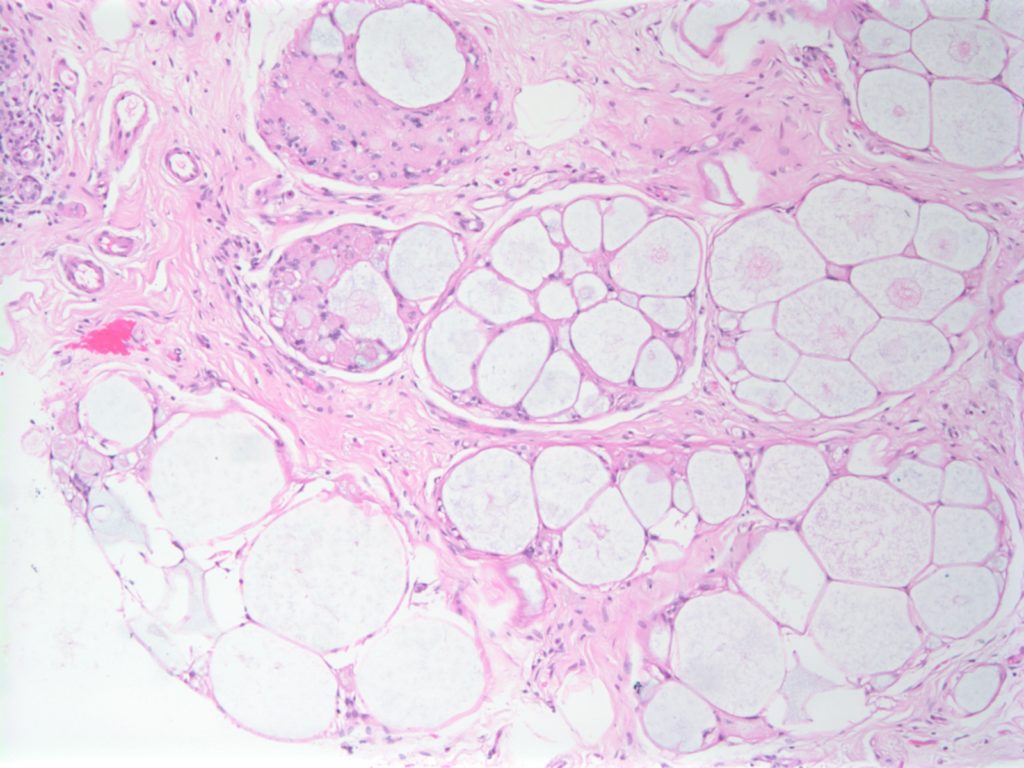
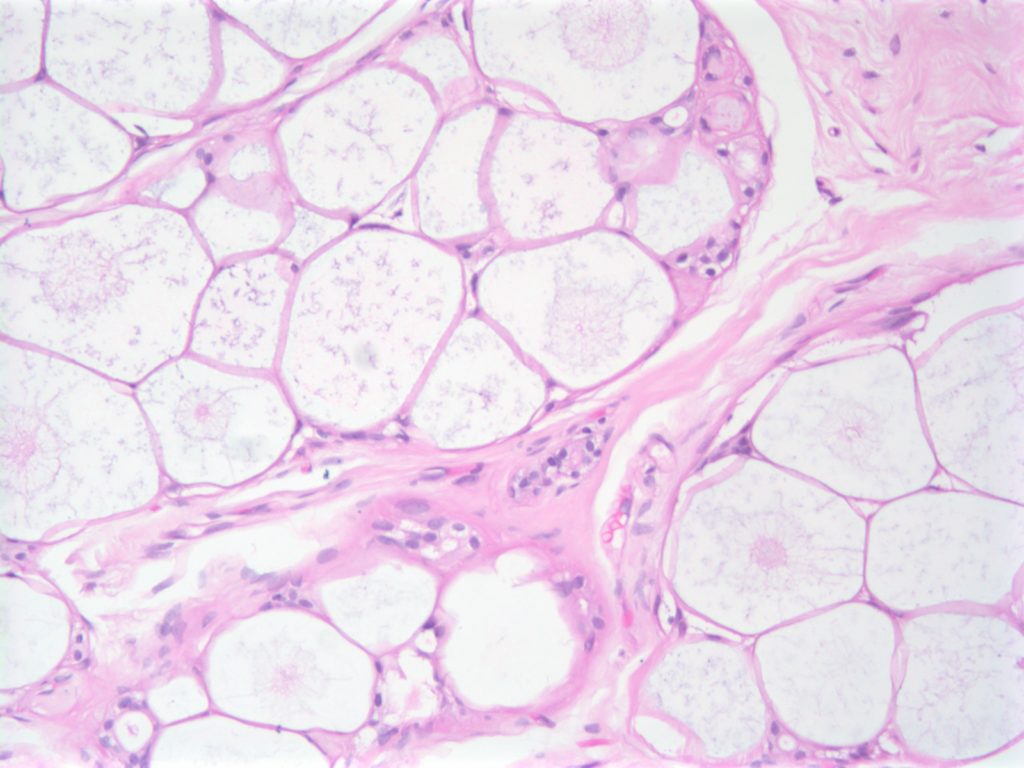
Resetkova, E., et. al. “Collagenous spherulosis of breast: morphologic study of 59 cases and review of the literature.”. Am J Surg Pathol. 30 (1):207. Jan 2006. doi:10.1097/01.pas.0000179237.91515.81
Fat necrosis may sometimes present as a palpable mass, which may raise clinical concern or distress in the patient. Approximately 1/2 of cases have a history of trauma. Fat necrosis is also a common finding in excision specimen after a preceding biopsy.
The characteristic findings of fat necrosis include mixed inflammation (neutrophils, histiocytes, etc.), fibroblastic proliferation, and liquefactive fat necrosis. Each of the component may vary in prominence. The most important thing is not to mistake fat necrosis for malignancy, and not miss an area of malignancy masked by fat necrosis (AE1/AE3 or CAM5.2 may be helpful in such situations).



There are many features of breast disease that encompass a wide spectrum of morphologic and radiologic findings, which have a varying risk of subsequent carcinoma. Some of these entities are thought to be direct precursors to invasive carcinoma, and others just signify a component of future risk of invasive carcinoma.
There are multiple situations where immunohistochemistry can serve an important role in breast disease. These include: differentiation of lesion types (e.g. UTH vs. ADH), identifying invasion (e.g DCIS vs. DCIS with invasion), and predictive/prognostic information (e.g. ER/PR/HER-2).
The following table and figure illustrate the risk of developing an invasive breast carcinoma based on the type of lesions previously identified. This information does not take into consideration other factors such as race and family history (complicated topic).
|
Relative
Risk
|
Absolute
Risk
(lifetime)
|
Breast
Lesion
|
|
1
|
3%
|
|
|
1.5 – 2
|
5-7%
|
|
|
4 – 5
|
13-17%
|
|
|
8 – 10
|
25-30%
|
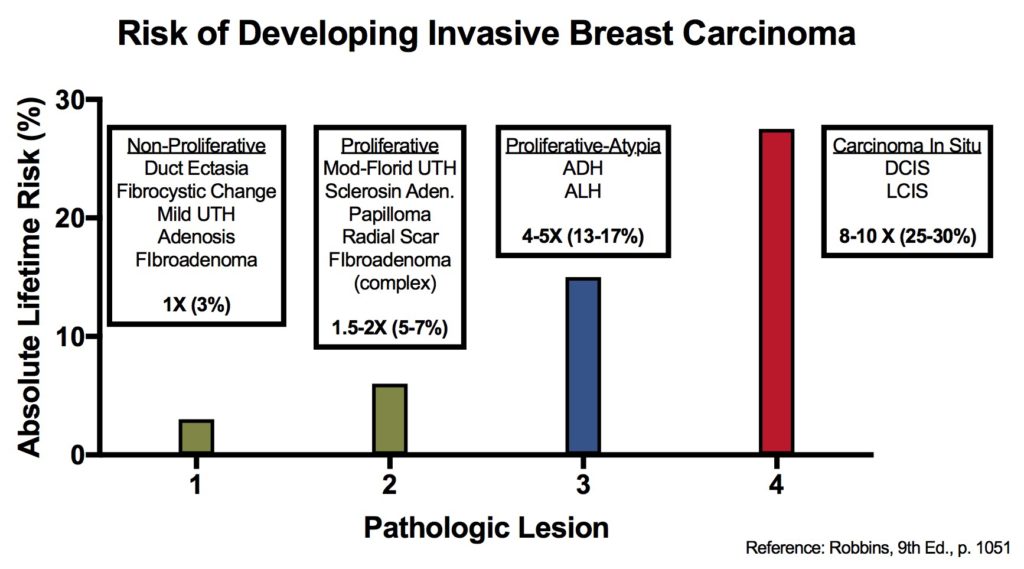
Kumar, Vinay, Abul K. Abbas, and Jon C. Aster. Robbins and Cotran Pathologic Basis of Disease. Ninth edition. Philadelphia, PA: Elsevier/Saunders, 2015.
|
|
Endocervical
Adenocarcinoma
|
Endometrial
Adenocarcinoma
|
|
Negative (7-8%+)
|
Positive (70-93%)
|
|
|
Positive (65-95%)
|
Usually Negative
|
|
|
Negative (4-20%+, 38% weak)
|
Strong Positive (67-90%)
|
|
|
Strong & Diffuse Positive (90-100%)
|
Patchy Positive cells (~35%)
|
|
|
HPV
|
Positive (67%)
|
Negative
|
|
IHC Marker
|
Adenocarcinoma
|
Mesothelioma
|
|
8%
|
100%
|
|
|
CK5/6 (CK5)
|
2%
|
100%
|
|
0%
|
93%
|
|
|
Thrombomodulin
|
14%
|
61-77%
|
|
N-Cadherin
|
30%
|
73%
|
|
93-100%
|
8%
|
|
|
80-100%
|
18%
|
|
|
BG8
|
96%
|
7%
|
|
81-88%
|
0%
|
|
|
84%
|
0%
|
|
|
72-74%
|
0%
|
|
|
72-85%
|
0%
|
|
CK7 is expressed in a majority of urothelial carcinomas. (CK7+/CK20+) Bladder adenocarcinomas with intestinal differentiation may lose CK7 expression.
|
|
|
(++/-) Wide variation in reported expression (15-97%), but appears a majority are positive (CK7+/CK20+). There is some evidence that CK20 may be helpful in CIS dx. Reactive urothelium shows CK20 expression in the umbrella cells. Most dysplasia/CIS cases show at least focal transmural CK20 expression. Sensitivity and specificity for CK20 have been shown to be >70% and >90%, respectively. (Ki-67. p53, & p16 may also be useful)
|
|
|
p63 staining is found in >90% of urothelial layer nuclei. PSA combined with p63 may be a helpful combination to differentiate a primary prostate tumor from urothelial carcinoma.
|
|
|
34betaE12
CK5/6
|
HMWK which is expressed in the basal layer of prostate glands, and urothelium (highly sensitive). Practically any tumor with squamous differentiation will usually express HMWKs.
|
|
p53
|
Expression found in 40-60% of bladder carcinomas (worse prognosis). Expression of p53 in >50% of cells is seen in CIS, whereas reactive urothelium show weak patchy nuclear staining.
|
|
Increased expression in flat CIS and low grade tumors.
|
|
|
CD44
|
May be useful in that it is normally expressed in the basal layer, and is absent in full thickness CIS.
|
|
Often positive in urothelial dysplasia.
|