Plasma Cell myeloma (PCM) is a disorder of monoclonal plasma cells, which is almost always associated with a monoclonal immunoglobulin protein in the serum and/or urine along with end organ damage (e.g. lytic bone lesions, renal failure, etc.).
Category Archives: Organ Systems
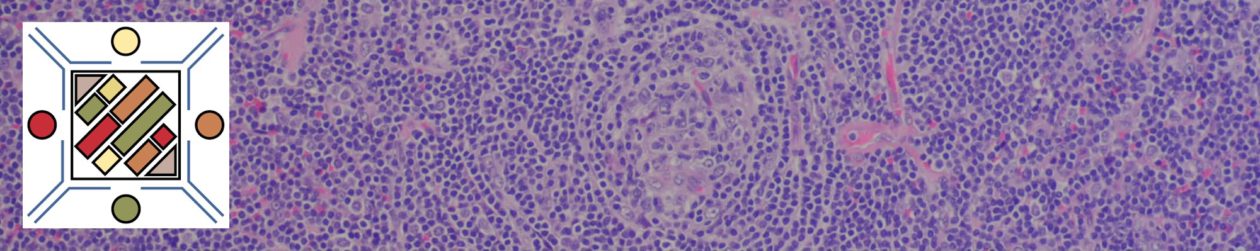
Diffuse Large B-Cell Lymphoma (DLBCL)
DLBCL is the most common non-Hodgkin lymphoma in the US (25,000 new cases/year) and is an intermediate grade lymphoma consisting of a heterogeneous group of mature B-cell lymphoid neoplasms sharing the common characteristics of diffuse architectural pattern and (medium or large) neoplastic lymphoma cells (nuclei >2x normal lymphocyte or equal to / exceeding a macrophage nuclei). DLBCL lymphoma can arise de novo or as transformation from another lymphoma (e.g. follicular lymphoma, CLL/SLL, marginal zone lymphoma).
Small Round Blue Cell Tumor
The small round blue cell tumor differential includes a wide variety of neoplasms, which in their purest form are morphologically indistinguishable from each other, and are dependent upon IHC and/or molecular studies for classification. The differential diagnosis includes: carcinoma, melanoma, lymphoma, rhabdomyosarcoma, PNET, and desmoplastic small round blue cell tumor.
An easy way to remember the IHC panel is to look at one’s hand. Each finger is a stain, and the thumb is always CD99. The 5 stain panel includes: AE1/AE3, S-100, CD45, Desmin, and CD99.
|
Stain
|
Comments
|
|
AE1/AE3 positivity only is c/w a carcinoma, and f/u with a CK7/CK20 profile panel and neuroendocrine markers (chromo. A, synaptophysin, CD56) is recommended. Co-expression of AE1/AE3 and desmin in the SRBCT setting is c/w a desmoplastic small round blue cell tumor. Co-expression of CD99 and AE1/AE3 may also suggest a poorly differentiated synovial sarcoma.
|
|
|
S-100 positivity is suggestive of a melanoma. Follow-up with melanoma specific markers is recommended (HMB-45, MART-1, etc.).
|
|
|
CD45 expression is c/s a lymphoma, and appropriate follow-up markers (CD3, CD20, etc.) is recommended.
|
|
|
Desmin positivity in isolation in the SRBCT setting is c/w a rhabdomyosarcoma (Myogenin or Myo-D1+ and WT1=) or Wilms Tumor (Myogenin and Myo-D1= and WT1+).
|
|
|
CD99 expression only is c/w a EWS/PNET. It should be noted that almost any other SRBCT may also express CD99 in some cases, and interpretation of CD99 in isolation, without AE1/AE3, Desmin, CD45, and S-100 is NOT recommended.
|
This 5 stain panel is only a screening panel, and more specific stains need to be followed up (e.g. HMB-45 = melanoma, myogenin = rhabdomyosarcoma, CK7/CK20 to further classify carcinomas). An important note, CD99 is the “thumb” because the stain should only be used with the other 4 stains in the panel. While CD99 is fairly sensitive for PNETs, it is NOT specific, and almost everything else on the differential list has been shown to occasionally stain with CD99. If all five markers on the screening panel are negative, then one may consider several possibilities: neuroblastoma (neuroendocrine marker +), lymphoproliferative d/o not expressing CD45 (leukemia, etc. – add CD43), or a carcinoma without AE1AE3 expression (use a second pan keratin marker with a differing cocktail, e.g. CAM5.2).
References
Arch Pathol Lab Med. Vol 132, March 2008.
Kandukuri SR, Lin F, Gui L, Gong Y, Fan F, Chen L, et al. Application of Immunohistochemistry in Undifferentiated Neoplasms: A Practical Approach. Arch Pathol Lab Med. 2017;141: 1014–1032. doi:10.5858/arpa.2016-0518-RA
Prostate – IHC Stains
Prostate Adenocarcinoma
IHC Expression Characteristics
|
Negative
|
|
|
Typically negative, rarely positive.
|
|
|
Racemase enzyme expressed by high grade PIN and Prostate adencoarcinoma (NOT a specific marker for prostate tissue). Relatively good specificity (~90%), but may mark benign prostate lesions. Often used in a cocktail with p63 and HMWKs (e.g. PIN4)
|
|
|
>95% sensitivity for prostate adenocarcinoma (highly specific)
|
|
|
PSAP
|
Earlier marker than PSA (now used occasionally as a backup) with less specificity compared to PSA
|
|
Combination stain containing a cocktail of AMACR (red), p63, and high molecular weight cytokeratins (CK5 & CK14)
|
|
|
34BetaE12
|
HMWK that is often used to highlight the basal layer of prostate glands (positivity means it is not invasive adenocarcinoma).
|
|
HMWK which has similar characteristics as 34betaE12
|
|
|
Nuclear marker which highlights the basal layer of benign prostate glands.
|
Prostatic Duct Carcinoma
IHC expression is the same as prostate adenocarcinoma. Morphology is similar to breast DCIS. Sometimes it may be confused with a primary colon adenocarcinoma (CDX-2+) or a primary urothelial carcinoma (PSA=).
References
Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. [edited by] DJ Dabbs. 3rd Edition. Elsevier, 2010.
Breast – Predictive Markers
One of the most important aspects of breast cancer diagnosis is the evaluation of therapeutic markers (ER, PR, and HER2). Ki-67 is often included in the panel as a prognostic marker. ER expression determines eligibility to receive hormonal therapy (Tamoxifin), PR expression is a prognostic marker, and HER-2 over-expression determines eligibility to receive Herceptin®. Diagnostically, the challenge is to consistently and accurately perform and interpret these IHC markers.
Estrogen Receptor (ER):
- Nuclear Marker
- Stain is reported as PERCENT STAINING OF TUMOR CELLS and STAIN INTENSITY (1+, 2+, 3+).
- 1% or greater nuclear expression in tumor cells is considered positive, and therefore eligible to receive hormonal therapy.
- CAP-ASCO recommendations are for <1 hr. from time of excision/biopsy to having a cut edge of tumor in 10% neutral bufferedormalin fixative. Fixation window of 6-72 hrs. These times should be noted in the pathology report (time of excision, time in gross room, and time in fixative).
- Negative staining results in biopsy material without an internal control should be repeated on the excisional specimen using blocks with both tumor and benign breast parenchyma.
Progesterone Receptor (PR):
- Nuclear Marker
- Stain is reported as PERCENT STAINING OF TUMOR CELLS and STAIN INTENSITY (1+, 2+, 3+).
- 1% or greater nuclear expression in tumor cells is considered positive.
- PR expression is a prognostic marker, and not directly used for eligibility to receive a specific treatment.
- PR expression without ER expression should raise significant concern that the ER and PR slides have been mixed up, or there is a problem with the ER assay. Many scientists believe that ER expression is required for PR expression.
HER-2 Overexpression (HER-2):
- Membraneous stain
- Stain is interpreted by combining stain intensity and percentage of tumor involvement to classify as (0, 1+, 2+, or 3+).
-
- 0 (negative) = No staining or cell membrane staining in <10% of tumor cells.
- 1+ (negative) = Faint membrane staining (partial membrane staining) in >10% of tumor cells.
- 2+ (equivocal) = Weak to moderate complete membrane staining in >10% of tumor cells, or strong complete staining in <10% of invasive tumor cells.
- 3+ (positive) = Strong complete membrane staining in >10% of tumor cells.
- CAP-ASCO recommendations are for <1 hr. from time of excision/biopsy to having a cut edge of tumor in 10% neutral buffered fomalin fixative. Fixation window of 6-72 hrs. Over-fixation is probably not a clinically significant issue practically, but given the absence of relevant IHC data and the highly regulated environment surrounding HER2 testing, f/u FISH testing for negative results (outside the fixative window) is necessary.
- Equivocal (2+) results should be followed-up with FISH testing, if IHC is used as the initial testing modality (most common). Less than 1/3rd of equivocal cases show Her2 over-expression by FISH analysis.
References
Hammond ME, et. al. “ASCO-CAP Guideline Recommendations for IHC Testing of ER and PR in Breast Cancer”. Arch Pathol Lab Med-Vol. 134, June 2010.
Wolff, A. C., Hammond, M. E. H., Hicks, D. G., Dowsett, M., McShane, L. M., Allison, K. H., et al. (2013). Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Archives of pathology & laboratory medicine. doi:10.5858/arpa.2013-0953-SA
Tafe, L. J., Janjigian, Y. Y., Zaidinski, M., Hedvat, C. V., Hameed, M. R., Tang, L. H., et al. (2011). Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Archives of pathology & laboratory medicine, 135(11), 1460–1465. doi:10.5858/arpa.2010-0541-OA
Arch Pathol Lab Med. 2001;125:746.
Breast – IHC Specific Markers
IHC stain expression pattern for various IHC antibodies in breast carcinoma.
|
IHC Stain
|
Comments
|
|
50-70%
|
|
|
30-60%
|
|
|
>80%
|
|
|
<10%
|
|
|
10-25%
|
|
|
0%
|
|
|
0%
|
|
|
0%
|
|
|
Mesothelin
|
<10%
|
Clin Cancer Rs 2005;11(10) May 15, 2005.
When evaluating for a possible breast primary, it is usually part of a larger differential diagnosis. CK7 and CK20 is the common starting point for carcinomas of unknown primary site (CUPS), and breast has a characteristic CK7+/CK20= profile. Unfortunately, this pattern is not uncommon, and more specific markers need to be performed. Two specific breast markers are GCDFP-15 and ER., but their sensitivity while good is limited. GATA-3 is a relatively newer antibody, which shows excellent sensitivity with good specificity, and should be strongly considered to be part of an antibody panel in work-up of potential breast carcinoma cases.
- GCDFP-15 is very specific for breast carcinoma in the setting of CUPS, but limited sensitivity.
- ER expression may be highly suggestive of a breast primary (especially epidemiologically), but it is not as specific.
- Other female organs (ovary/uterus) not uncommonly express ER, and practically any tissue can occasionally excess ER.
- GATA-3 appears to have excellent sensitivity (>90%) with good (not perfect) specificity.
Liu, et al (Biocare Medical, Concord, CA)
|
Tumor
|
GATA-3
|
GCDFP-15
|
MGB
|
|
Breast Carcinoma
|
94%
|
35-55%
|
65-70%
|
|
ER-negative breast ca.
|
69%
|
15%
|
35%
|
|
Urothelial Carcinoma
|
86%
|
|
|
Breast Metastasis vs. Ovarian Ca. Primary
|
Tumor
|
WT-1
|
CA-125
|
GCDFP-15
|
|
Primary Ovarian Ca.
(N=41)
|
76%
|
73%
|
0%
|
|
Metastatic Breast Ca.
(N-40)
|
3%
|
10%
|
43%
|
|
P-Value
|
<0.001
|
<0.001
|
<0.001
|
Chen, USCAP, 2004
References
Liu, H., Shi, J., Wilkerson, M. L., & Lin, F. (2012). Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. American Journal of Clinical Pathology, 138(1), 57–64. doi:10.1309/AJCP5UAFMSA9ZQBZ
Miettinen, M., McCue, P. A., Sarlomo-Rikala, M., Rys, J., Czapiewski, P., Wazny, K., et al. (2014). GATA3: A Multispecific But Potentially Useful Marker in Surgical Pathology: A Systematic Analysis of 2500 Epithelial and Nonepithelial Tumors. The American Journal of Surgical Pathology, 38(1), 13–22. doi:10.1097/PAS.0b013e3182a0218f
Clin Cancer Rs 2005;11(10) May 15, 2005.
Chen, USCAP, 2004
Breast – DCIS vs. LCIS
Lobular vs. ductal differentiation in breast lesions can on occasion be problematic. E-cadherin is well described as a marker of ductal differentiation, but aberrant expression has be reported in 2-16% of cases of lobular neoplasia. Some authors suggest not changing the interpretation/diagnosis based on expression of E-cadherin is the morphology is consistent with lobular carcinoma. This author is not sure why one would do the stain to begin with if the morphologic characteristics are consistent with lobular carcinoma. 34betaE12 (CK903) has been described as a sensitive and specific in showing perinuclear dot-like expression in cases of lobular carcinoma and negativity in ductal carcinomas. The combined panel of 34betaE12 and E-cadherin makes a nice complimentary panel to evaluate between lobular and ductal differentiation in breast carcinomas when morphology alone is not clear.
|
IHC Stain
|
DCIS
|
LCIS
|
|
E-Cadherin
|
+
|
=
|
|
34betaE12
|
=
|
+
|
References
Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138(12):1629–1642. doi:10.5858/arpa.2014-0094-RA.
Bratthauer GL, Moinfar F, Stamatakos MD, et al. Combined E-cadherin and high molecular weight cytokeratin immunoprofile differentiates lobular, ductal, and hybrid mammary intraepithelial neoplasias. Hum Pathol. 2002;33(6): 620–627.
Breast – Stromal Invasion vs. In Situ or Benign Glands
Not infrequently in breast pathology, the differential diagnosis of micro-invasion vs. DCIS or a scerlosing lesion +/- tubular carcinoma will occur. These type of cases can be very challenging based just on H&E morphology. Fortunately, there are multiple IHC markers, which can be helpful in identifying the myoepithelial layer. The main pitfall in interpretation of most of these markers, is that they will also mark myofibroblasts (on occasion) in the intervening stroma. When this occurs, and myofibrilblasts abut the glands, the staining pattern can be misinterpreted as an intact myoepithelial layer.
|
Antibody
|
MEC
|
Myofibroblasts
|
|
++++
|
++
|
|
|
++++
|
++
|
|
|
SMA
|
++++
|
+++
|
|
p63 (nuclear)
|
++++
|
–
|
|
++++
|
–
|
|
|
+++
|
+
|
|
|
++
|
–
|
SMA = Smooth Muscle Action, SMM-HC = Smooth Muscle Myosin Heavy Chain.
The most commonly used stains include SMM-HC, calponin, CK5, and p63. p63 can be combined with any of the other cytoplasmic stains as part of a double stain protocol. SMA is the most proned of the stains to also mark fibroblasts. S-100 is NOT recommended as a myoepithelial marker. p63 has the least association with myofibroblast staining, but expression may be discontinuous in the myoepithelial cells layer, which can lower sensitivity when used alone. In the opinion of many breast pathologists, SMM-HC as a single stain probably has the best combined sensitivity for the myoepithelial cells with minimal myofibroblastic staining, but many will use multiple markers in difficult cases.
Myofibroblast Staining
Actin > Calponin > SMM-HC
References
Hicks DG. Immunohistochemistry in the diagnostic evaluation of breast lesions. Appl Immunohistochem Mol Morphol. 2011;19(6):501–505. doi:10.1097/PAI.0b013e31822c8a48.
Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138(12):1629–1642. doi:10.5858/arpa.2014-0094-RA.
Breast – UDH vs. ADH
A common differential diagnosis in breast lesions is between usual type hyperplasia (UDH) and atypical ductal hyperplasia (ADH)/ductal carcinoma in situ (DCIS). In difficult cases there are some immunohistochemical patterns, which may be helpful to differentiate between diagnoses. UDH has mosaic expression pattern with HMWK (high molecular weight keratins) (e.g. CK5 or CK5/6) whereas ADH/DCIS typically does not. ADH/DCIS does typically has strong uniform up regulation of estrogen receptor (ER) in contrast to UDH.
DIAGNOSTIC FEATURES
Cellular Population
|
Florid UDH
|
ADH
|
|
True hyperplasia contains a mixture of cell types (streaming, slit-like spaces)
|
Clonal population of monotonous cells with rigid “punched-out” spaces
|
CK Expression
|
Florid UDH
|
ADH
|
|
Mixture of basal cells (CK5/14/17) and luminal cells (CK7/8/18)
|
Monotonous population of luminal cell types (CK 7/8/18)
|
ER Expression
|
Florid UDH
|
ADH
|
|
Variable patchy expression
|
Usually uniform strong expression
|
Breast Cancer Relative Risk
|
Florid UDH
|
ADH
|
|
Slightly increased (1.5-2 X)
|
Moderately increased (3.7-5.3 X)
|
|
Diagnostic Features
|
Florid UDH
|
ADH
|
|
Cellular Population
|
True hyperplasia contains a mixture of cell types (streaming, slit-like spaces)
|
Clonal population of monotonous cells with rigid “punched-out” spaces
|
|
CK Expression
|
Mixture of basal cells (CK5/14/17) and luminal cells (CK7/8/18)
|
Monotonous population of luminal cell types (CK 7/8/18)
|
|
EP Expression
|
Variable patchy expression
|
Usually uniform strong expression
|
|
Breast Cancer R.R.
|
Slightly increased (1.5-2X)
|
Moderately increased (3.7-5.3X)
|
References
Hicks DG. Immunohistochemistry in the diagnostic evaluation of breast lesions. Appl Immunohistochem Mol Morphol. 2011;19(6):501–505. doi:10.1097/PAI.0b013e31822c8a48.
Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138(12):1629–1642. doi:10.5858/arpa.2014-0094-RA.
Breast – Normal IHC Expression
Normal breast ducts and lobules are lined by a 2-cell layer composed of luminal and myoepithelial cells. There are also interspersed “basal” cells, which probably represent the epithelial progenitor cells.
|
IHC Marker
|
Luminal Cells
|
Myoepithelial Cells
|
|
LMWCKs
(CK7/8/18)
|
Positive
|
Negative
|
|
Variable Expression
|
Negative
|
|
|
HMWCKs
(CK5/14/17)
|
Negative
|
Positive
|
|
SMA
|
Negative
|
Positive
|
|
Negative
|
Positive
|
|
|
Negative
|
Positive
|
SMA=Smooth Muscle Actin, SMM-HC=Smooth Muscle Myosin Heavy Chain
An understanding of the normal IHC expression pattern in breast ductal tissue is important when considering IHC use in the differential diagnosis of breast pathology.
References
Hicks DG. Immunohistochemistry in the diagnostic evaluation of breast lesions. Appl Immunohistochem Mol Morphol. 2011;19(6):501–505. doi:10.1097/PAI.0b013e31822c8a48.
Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138(12):1629–1642. doi:10.5858/arpa.2014-0094-RA.