Scattered linear fibers without intersections. Normal bone marrow.
Category Archives: Organ Systems
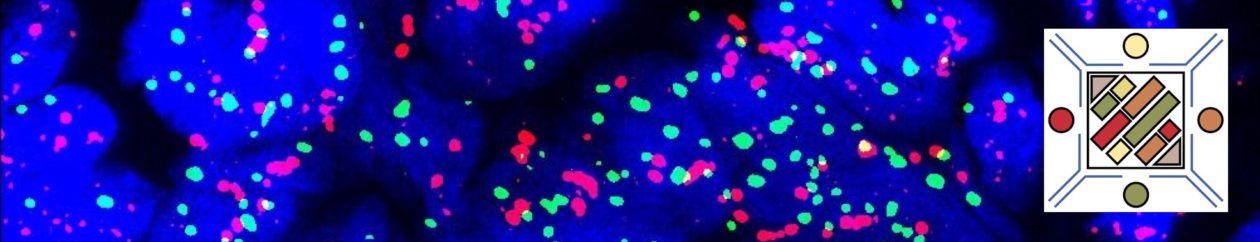
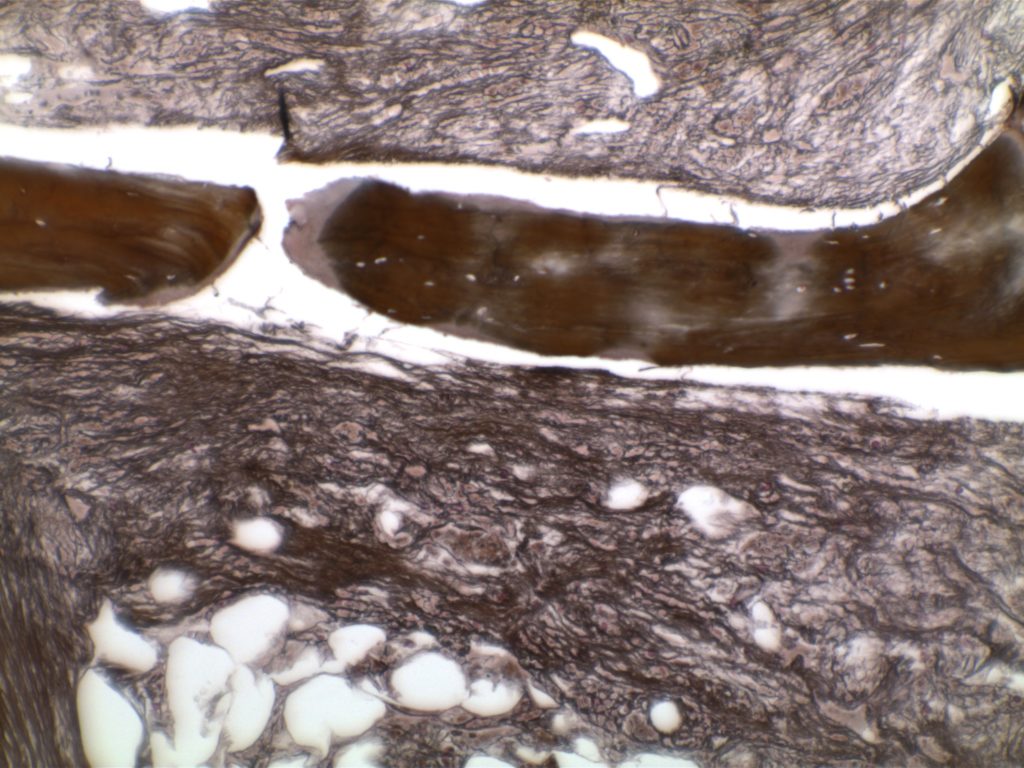
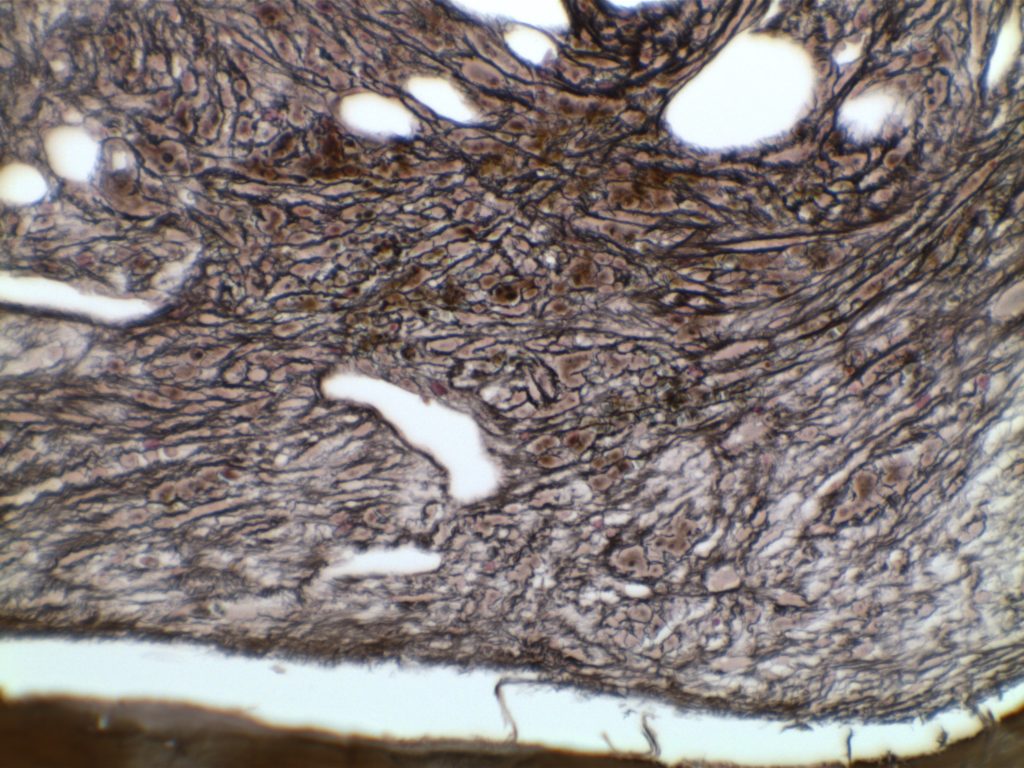
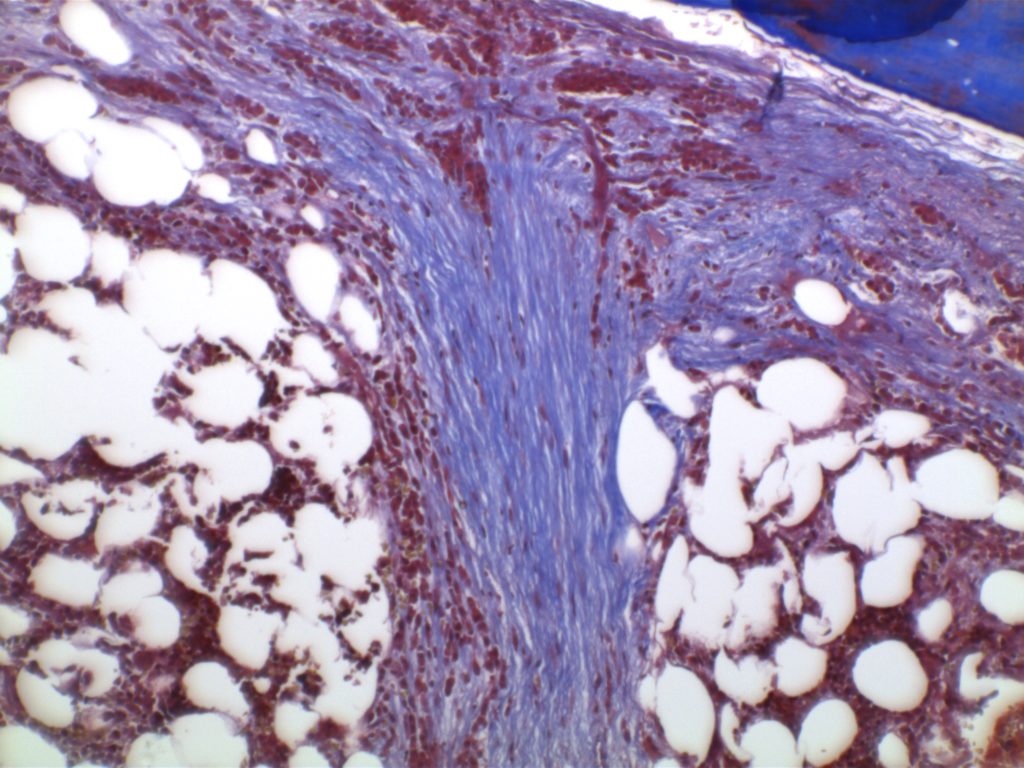
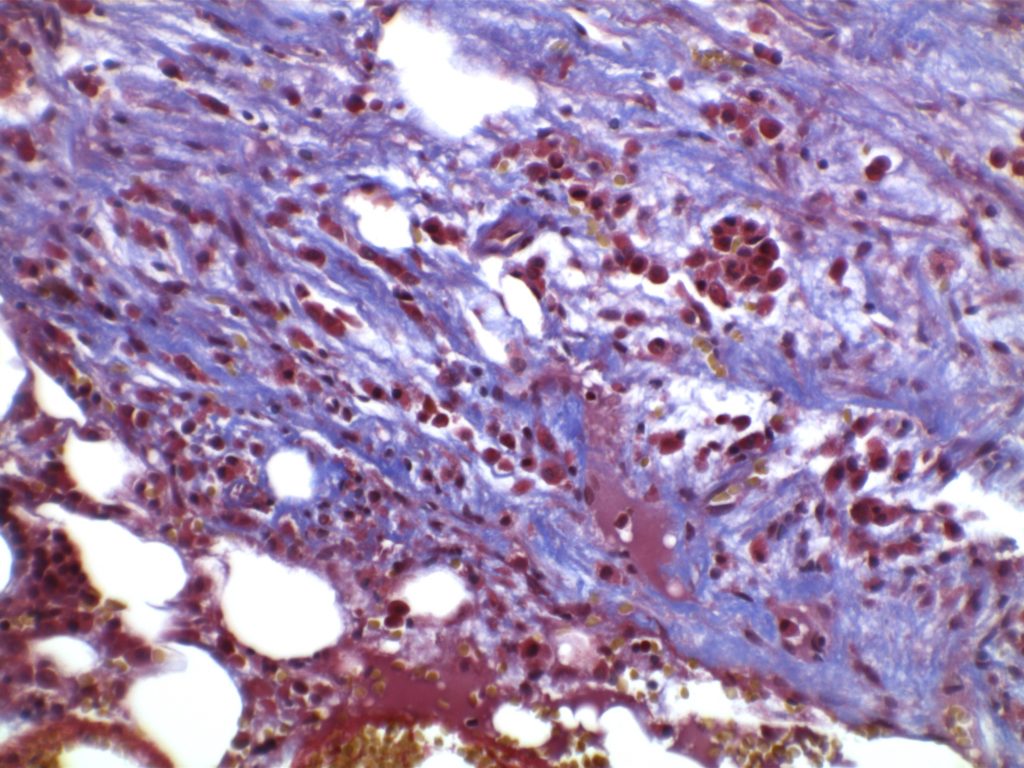
MF3 – Reticulin Fibrosis
Diffuse increase of reticulin fibers with increased density and numerous intersections. Increased thick bundles of fibers consistent with collagen fibrosis. Osteosclerosis usually present.
Photomicrographs
References
MDS Cytogenetics
|
-7 or del (7q)
|
-5 or del(5q)
|
|
i(17q) or t(17p)
|
-13 or del(13q)
|
|
del(11q)
|
del(12p) or t(12p)
|
|
del(9q)
|
idic(X)(q13)
|
|
t(11;16)(q23;p13.3)
|
t(3;21)(q26.2;q22.1)
|
|
t(1;3)(p36.3;q21.2)
|
t(2;11)p21;q23)
|
|
inv(3)(q21q26.2)
|
t(6;9)(p23;q34)
|
Breast Myofibroblastoma
Myofibroblastoma (MFB) of the breast is an uncommon but well described benign stromal neoplasm of the breast. MFB can have many different morphologic patterns (see variants below), but important defining characteristics include:
- Mesenchymal tumor without epimyoepithelial elements
- No necrosis
- Low proliferative activity (≤2 mitoses/10 hpf)
- No atypical mitoses
- Characteristic immunophenotype
Acute Undifferentiated Leukemia
Care should be taken that immunophenotyping panels are extensive, and consideration is given to other entities such as plasmacytoid dendritic precursors, non-hematopoietic tumors, NK-cell precursors, etc. Essentially, a diagnosis of exclusion.
Myeloid/Lymphoid Neoplasms Associated with Eosinophilia
- PDGFA
- Eosinophilia with increased tryptase and marrow mast cells
- Cryptic deletion in chromosome 4q12
- FIP1L1-PDGFA, many other partners
- Responsive to tyrosine kinase inhibitors (e.g. imatimib/Gleevec®)
- PDGFB
- Eosinophilia combined with monocytosis that mimics CMML
- t(5;12)(q31;33;p12) ETV6-PDGFB, many other partners
- Responsive to TKIs
- FGFR1
- PCM1-JAK2 (provisional entity)
- Eosinophilia along with acute lymphoblastic leukemia (B-cell) or lymphoma (T-cell)
- Bone marrow with lymphoid aggregates and increased/left shifted erythroid precursors (mimics PMF)
- May respond to JAK2 inhibitors (e.g ruxolitinib/Jakafi®)
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127: 2391–2405. doi:10.1182/blood-2016-03-643544
Swerdlow SH, Campo E, Harris, NL, Jaffe ES, Pileri SA, Stein H, Thiele J (Eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC: Lyon 2017
MGUS – IgM
IgM Monoclonal Gammopathy of Undetermined Significance
IgM MGUS is considered as a precursor to lymphplasmacytic lymphoma (LPL) and/or Waldenstrom and is separate from Non-IgM MGUS (IgG, IgA), which is a precursor to plasma cell myeloma.
MGUS – Non-IgM
Monoclonal Gammopathy of Uncertain Significance (MGUS)
- < 3 gm/dL serum M protein (and)
- <10% clonal bone marrow plasma cells (and)
- Asymptomatic patients, no end organ damage (CRAB: hypercalcemia, renal insufficiency, anemia, bone lesions)
- No evidence of other B-cell lymphoproliferative disorder
Smoldering (Asymptomatic) Myeloma
Smoldering (Asymptomatic) Myeloma
- >3 gm/dL serum M protein (or) urinary M protein ≥500 mg/24 hours
- (and/or) 10-60% bone marrow plasma cells (usually 10-20% plasma cells)
- Asymptomatic patients with no evidence of end-organ damage or biomarker evidence of malignancy (myeloma-defining events)
Plasma Cell Leukemia (PCL)
- >20% clonal plasma cells in the peripheral blood (PB)
- (or) >2,000/μL absolute count in PB