CD34 (human hematopoietic progenitor cell antigen) is expressed by endothelial cells and embryonic cells of the hematopoetic system. CD34 is used in a wide variety of ways in diagnostic pathology. It can generally be divided into hematopathology and non-hematopathology uses. As with most situations in diagnostic immunohistochemistry, CD34 is often best used as part of a targeted panel considering the differential diagnosis at hand.
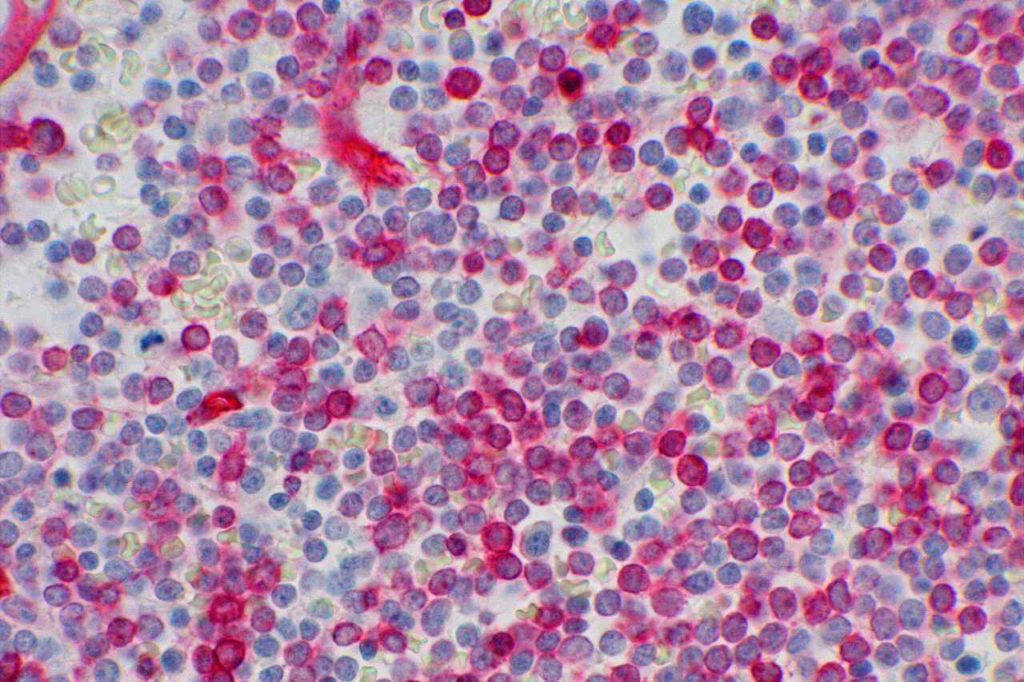
Hematopathology– CD34 will mark myeloblasts and a subset of lymphoblasts. It is therefore not specific for myeloid differentiation (although fairy sensitive). Normal myeloblasts will express CD34, and in cases of AML approximately 70% of cases will be positive. Approximately 1/3rd of cases of ALL may express CD34. It is also important to understand that not all blasts within a specific case may all express CD34, and correlation with flow cytometry data and aspirate count is critical. CD34 also stains vascular endothelium, which makes for a nice internal control in bone marrow biopsies. Granulocytic sarcomas (a.k.a. chloroma, soft tissue AML, or leukemia cutis) often have monocytic differentiation, and only ~5% of case demonstrate CD34 expression.
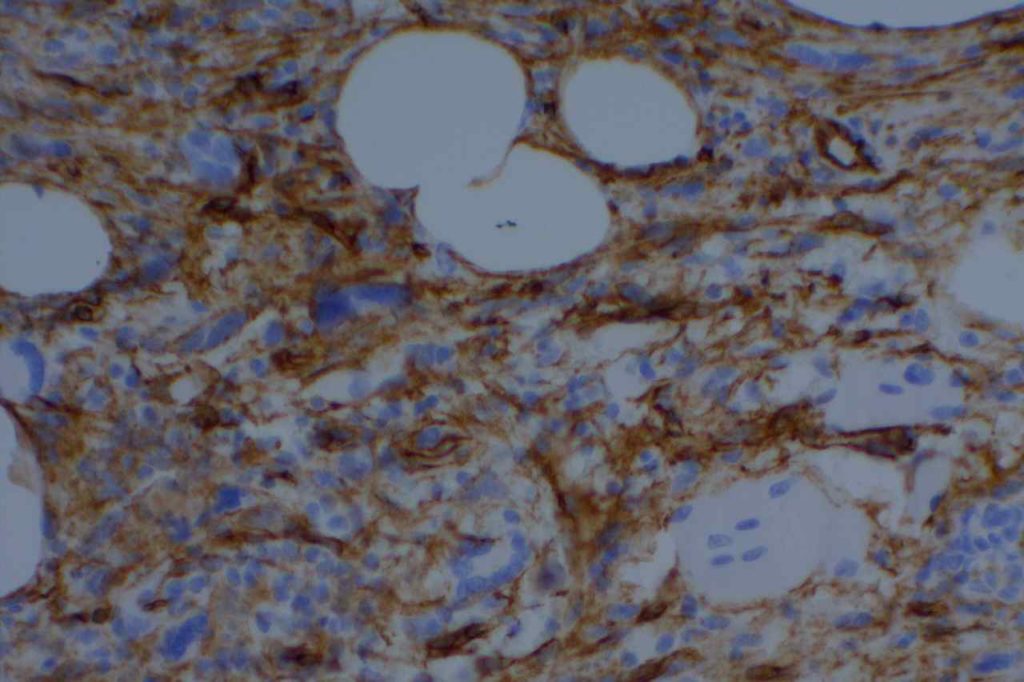
Non-Hematopathology– CD34 is expressed in cases of dermatofibrosarcoma protuberant (DFSP), hemangiopericytoma, solitary fibrous tumor, angiosarcoma & Kaposi’s sarcoma (>85%), epithelioid sarcoma (often), lymphocyte rich T cell lymphoma, gastrointestinal stromal tumors, and spindle cell lipomas. CD34 is not expressed in dermatofibromas, desmoplastic mesothelioma, & endometrial stromal sarcoma. CD34 is also an excellent vascular marker, which is helpful in identifying lymph-vascular invasion and tumors of vascular origin.
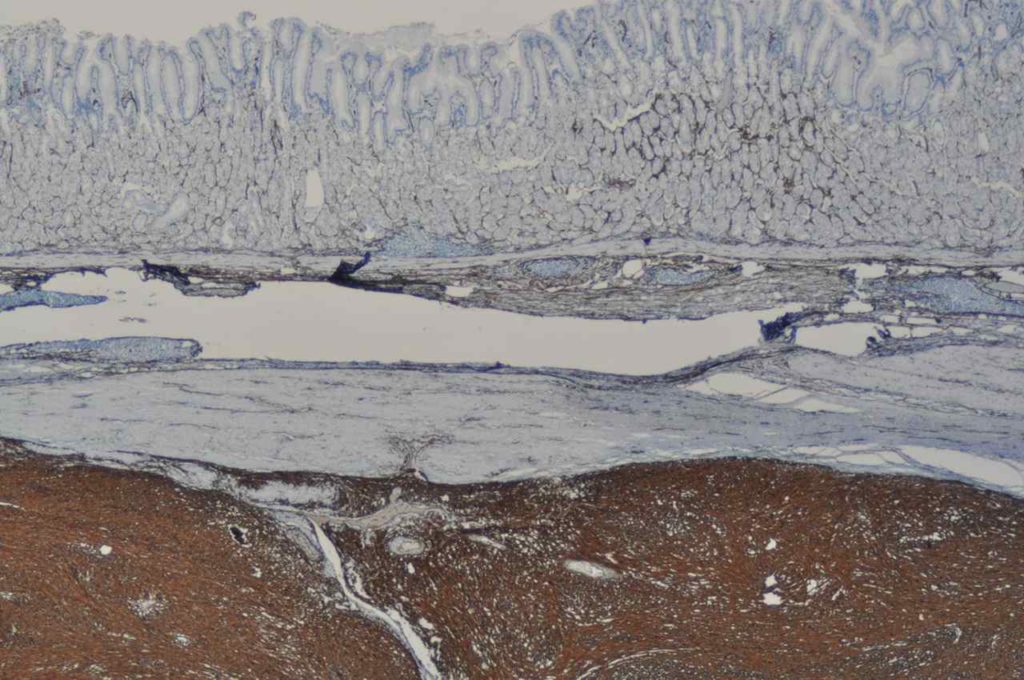
In hepatocellular carcinoma, CD34 highlights increased vascular structures associated with tumorigenesis. Normal liver only shows CD34 expression in portal vasculature and sinusoids immediate adjacent to portal tracts.
Lymphatic & Vascular Invasion – CD34 is sometimes utilized to evaluate for “lymphovascular invasion” (LVI) in different tumors (e.g. breast more commonly). CD34 and CD31 will stain both (blood) vascular endothelium (strong & sensitive) and also lymphatic endothelium (variable & less sensitive). Sometimes CD34 will stain stromal cells, which can mimic endothelium (false positive).
To determine the true nature of LVI (i.e. lymphatic vs. blood vascular invasion) additional markers specific for lymphatic endothelium (podoplanin or D2-40) need to be performed, and comparison made to CD31/CD34 for determination of invasion type:
- Lymphatic invasion – CD34/CD31 +/-, D2-40/podoplanin +
- Blood vascular invasion – CD34/CD31 +, D2-40/podoplanin –
CD34 Expression – Hematopathology
- Myeloblasts (~70% of cases of AML)
- Lymphoblasts (small subset)
- Endothelial Cells (blood vascular and lymphatic)
- Megakaryocytes (cytoplasmic)
CD34 Expression – Non-Hematopahtology
- Skin – Dermatofibrosarcoma Protuberans – molecular analysis for COL1A1-PDGFB can be performed in difficult cases.
- Skin – Superficial Acral Fibromyxoma (digital fibroma) – CD34+/CD99+/EMA+/- (cellular digital fibroma CD34+, CD99-/EMA-)
- Skin – Superficial CD34+ Fibroblastic Tumor
- Epithelioid Sarcoma (CD34+/CK+)
- Hemangiopericytoma
- Solitary Fibrous Tumor
- Vascular Neoplasms (e.g. Kaposi Sarcoma, Angiosarcoma, etc.)
- Normal Endothelium
- Gastrointestinal Stromal Tumor (~70%, some smooth muscle neoplasms can express CD34 – up to 10%)
- Spindle Cell Lipoma
- Epithelioid Sarcoma
- Subset of numerous other entities
Photomicrographs
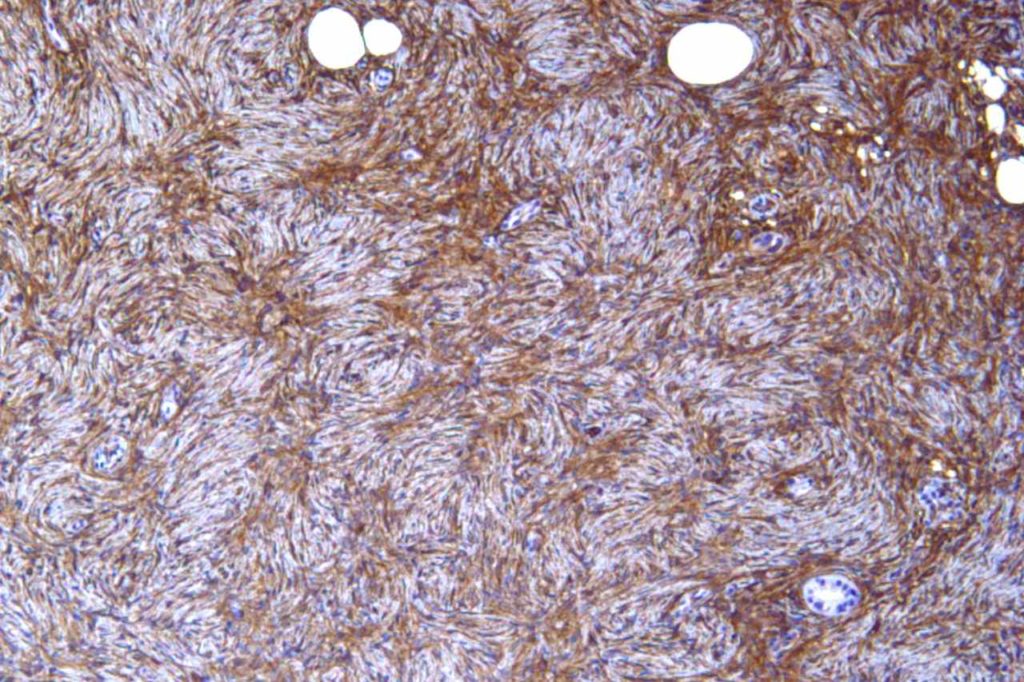
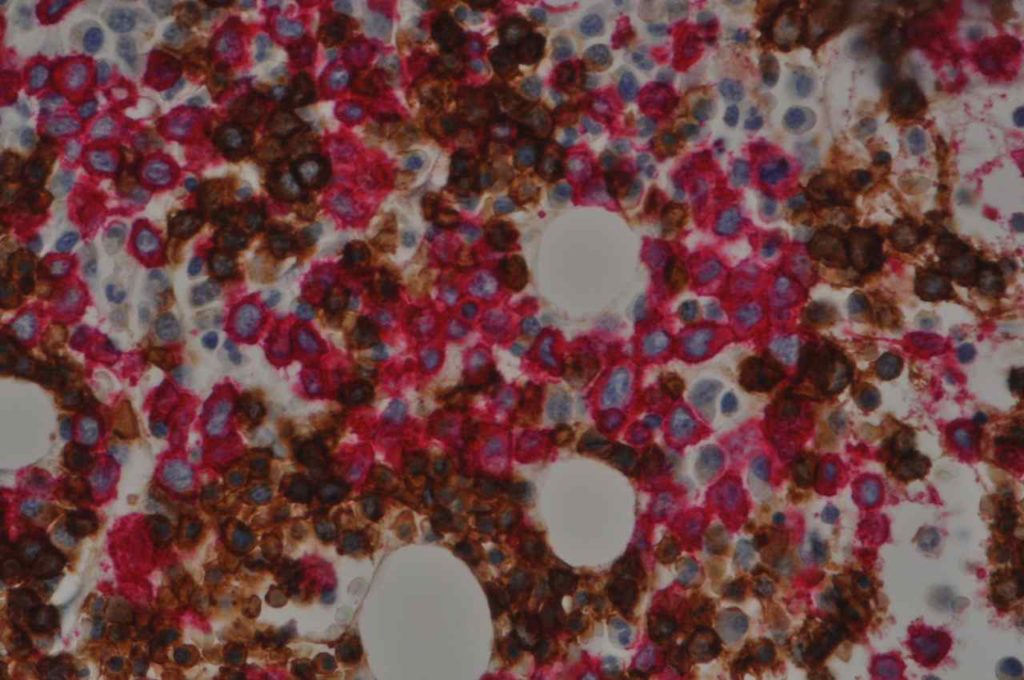
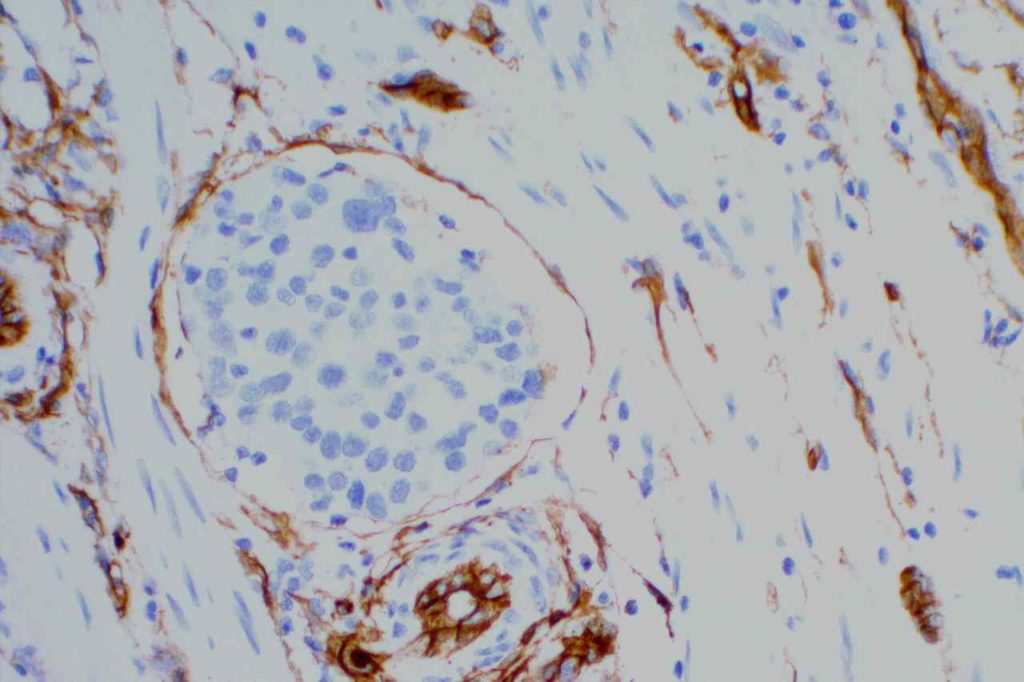
References
Mohammed RAA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31: 1825–1833. doi:10.1097/PAS.0b013e31806841f6
Yang H, Yu L. Cutaneous and Superficial Soft Tissue CD34(+) Spindle Cell Proliferation. Arch Pathol Lab Med. 2017;141: 1092–1100. doi:10.5858/arpa.2016-0598-RA
Rao N, Colby TV, Falconieri G, Cohen H, Moran CA, Suster S. Intrapulmonary solitary fibrous tumors: clinicopathologic and immunohistochemical study of 24 cases. Am J Surg Pathol. 2013;37: 155–166. doi:10.1097/PAS.0b013e31826a92f5
de Smet D, Trullemans F, Jochmans K, Renmans W, Smet L, Heylen O, et al. Diagnostic Potential of CD34+ Cell Antigen Expression in Myelodysplastic Syndromes. Am J Clin Pathol. 2012;138: 732–743. doi:10.1309/AJCPAGVO27RPTOTV
Rosado FGN, Itani DM, Coffin CM, Cates JM. Utility of immunohistochemical staining with FLI1, D2-40, CD31, and CD34 in the diagnosis of acquired immunodeficiency syndrome-related and non-acquired immunodeficiency syndrome-related Kaposi sarcoma. Arch Pathol Lab Med. 2012;136: 301–304. doi:10.5858/arpa.2011-0213-OA
Patil DT, Rubin BP. Gastrointestinal stromal tumor: advances in diagnosis and management. Arch Pathol Lab Med. 2011;135: 1298–1310. doi:10.5858/arpa.2011-0022-RA
Bénet C, Gomez A, Aguilar C, Delattre C, Vergier B, Beylot-Barry M, et al. Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. Am J Clin Pathol. 2011;135: 278–290. doi:10.1309/AJCPFMNYCVPDEND0
Dunphy CH, O’Malley DP, Perkins SL, Chang C-C. Analysis of immunohistochemical markers in bone marrow sections to evaluate for myelodysplastic syndromes and acute myeloid leukemias. Appl Immunohistochem Mol Morphol. 2007;15: 154–159. doi:10.1097/PAI.0b013e318030dec7
Dunphy CH, Polski JM, Evans HL, Gardner LJ. Evaluation of bone marrow specimens with acute myelogenous leukemia for CD34, CD15, CD117, and myeloperoxidase. Arch Pathol Lab Med. 2001;125: 1063–1069.
Wang HL, Kim CJ, Koo J, Zhou W, Choi EK, Arcega R, et al. Practical Immunohistochemistry in Neoplastic Pathology of the Gastrointestinal Tract, Liver, Biliary Tract, and Pancreas. Arch Pathol Lab Med. 2017;141: 1155–1180. doi:10.5858/arpa.2016-0489-RA
Wick, MR. “Immunohistochemical approaches to the diagnosis of undifferentiated malignant tumor.”Annals of Diagnostic Pathology12(2008):72-84.
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 88.