CDX-2 is a nuclear transcription marker that regulates differentiation and proliferation of intestinal epithelial cells, and also serves as tumor suppressor gene. CDX-2 is normally expressed in small and large intestine epithelium. It is a sensitive marker for colorectal adenocarcinomas (especially with strong diffuse expression). It can also mark non-colorectal GI adenocarcinomas, columnar cell variant of papillary thyroid carcinoma (and other variants of papillary thyroid carcinoma, classic and modular variant), ovarian mutinous carcinomas, bladder adenocarcinomas, goblet cells in Barrett’s esophagus, along with other tumors (data in tables below). Practically speaking, if a particular tumor can have any type of intestinal differentiation, then it most likely can have CDX-2 expression.
Pitfalls
It is important to note that antibody sensitivity can have significant inter laboratory variation due to both clone selection and antibody optimization (Borrisholt, et. al.). Each laboratory should have a good understanding of the performance characteristics of their CDX-2 clone and optimization.
IHC Survey of Primary and Metastatic Caricnomas (Werling, R. W., et. al.)
|
Tumor
|
Expression (%)
|
|
Gastric
Adencarcinoma
|
70% (N=24)
|
|
Pancreatic
Carcinoma
|
32% (N=22)
|
|
Cholangiocarcinoma
|
25% (N=16)
|
|
Carcinoid Tumor
|
42% (N=12)
|
|
Ovarian Carcinoma
(Mucinous)
|
64% (N=14)
|
|
Bladder
Adenocarcinoma
|
100% (N=2)
|
Common expression patterns in carcinoma (Dennis, J. L, et. al.)
|
Tumor
|
Expression (%)
|
|
Breast
|
0%
|
|
Colon
|
>90%
|
|
Lung
|
<5%
|
|
Pancreas
|
0-32%
|
|
Stomach
|
20-70%
|
|
Prostate
|
<5%
|
(Enriquez, M.L., et. al.)
|
Tumor
|
Expression (%)
|
|
Columnar Cell Variant
Papillary Thyroid Carcnioma
|
55% (N=11)
|
CDX-2 and Villin expression in human tumors (2-3+ expression) (Werling, R.W., et. al.)
|
Tumor Type
|
No.
|
CDX2
|
Villin
|
|
G.I. Tract
|
|
|
|
|
Colon Adenocarcinoma
|
75
|
99%
|
82% (n=73)
|
|
Duodenal Adenoma
|
10
|
100%
|
100%
|
|
Gastric Adenocarcinoma
|
24
|
70%
|
42%
|
|
Esophageal Adenocarcinoma
|
9
|
67%
|
78%
|
|
Pancreatic Adenocarcinoma
|
22
|
32%
|
40%
|
|
Cholangiocarcinoma/GB
|
16
|
25%
|
60%
|
|
Hepatocellular Carcinoma
|
12
|
0%
|
0%
|
|
Carcinoid Tumors
|
12
|
42%
|
34%
|
|
Ovary
|
|
|
|
|
Mucinous Adenocarcinoma
|
14
|
64%
|
64%
|
|
Mucinous Cystadenoma
|
13
|
8%
|
0%
|
|
Mucinous Borderline Tumor
|
4
|
25%
|
0%
|
|
Non-Mucinous
|
36
|
0%
|
0% (n=31)
|
|
Genitourinary Tract
|
|
|
|
|
Urothelial Carcinoma
|
21
|
0%
|
0%
|
|
Adenocarcinoma
|
2
|
100%
|
100%
|
|
Urachal Caricinoma
|
1
|
100%
|
100%
|
|
Renal Cell Carcinoma
|
7
|
0%
|
0% (n=3)
|
|
Prostate Adenocarcinoma
|
27
|
4%
|
0% (n=24)
|
|
Breast Carcinoma
|
34
|
0%
|
0%
|
|
Lung (45 primary; 18 met)
|
63
|
0%
|
5%
|
|
Adenocarcinoma
|
11
|
0%
|
0%
|
|
Squamous Cell Carcinoma
|
11
|
0%
|
0%
|
|
Non-Small Cell Carcinoma NOS
|
33
|
0
|
9%
|
|
Mucinous Carcinoma
|
2
|
0%
|
0%
|
|
Small Cell Carcinoma
|
4
|
0%
|
0%
|
|
Mesothelioma
|
7
|
0%
|
0%
|
|
Head and Neck
|
|
|
|
|
Thyroid
|
36
|
3%
|
0%
|
|
Papillary Carcinoma
|
11
|
9%
|
0%
|
|
Follicular Adenoma/Carcinoma
|
25
|
0%
|
0%
|
|
Salivary Gland
|
12
|
0%
|
0%
|
|
Mixed Tumor
|
6
|
0%
|
0%
|
|
Low Grade Carcinoma
|
6
|
0%
|
0%
|
|
Squamous Cell Carcinoma
|
13
|
0%
|
0% (n=11)
|
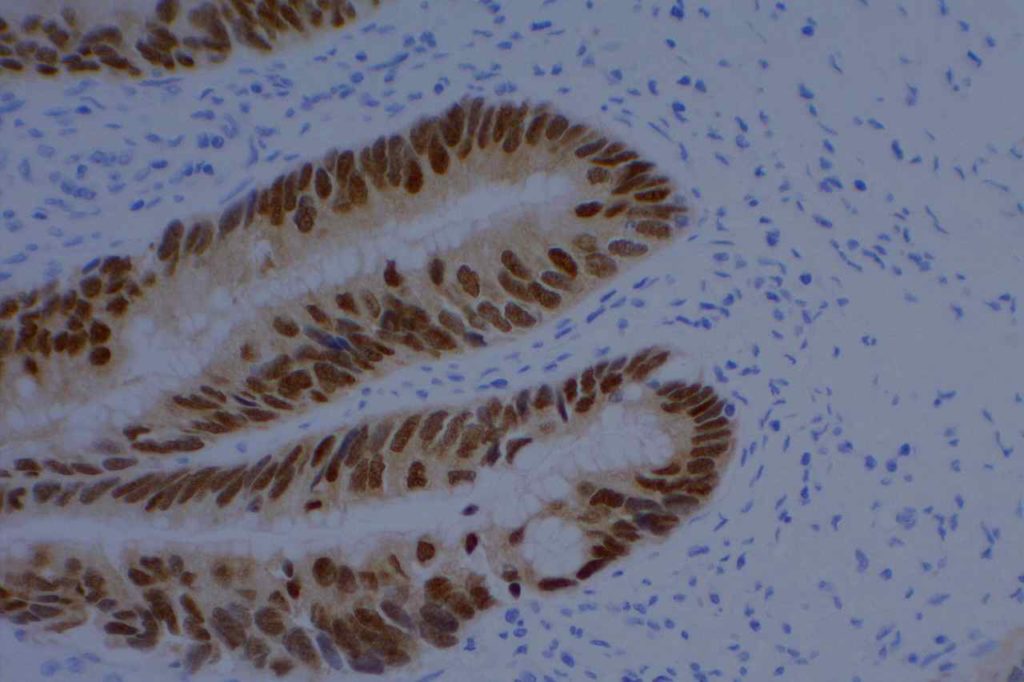
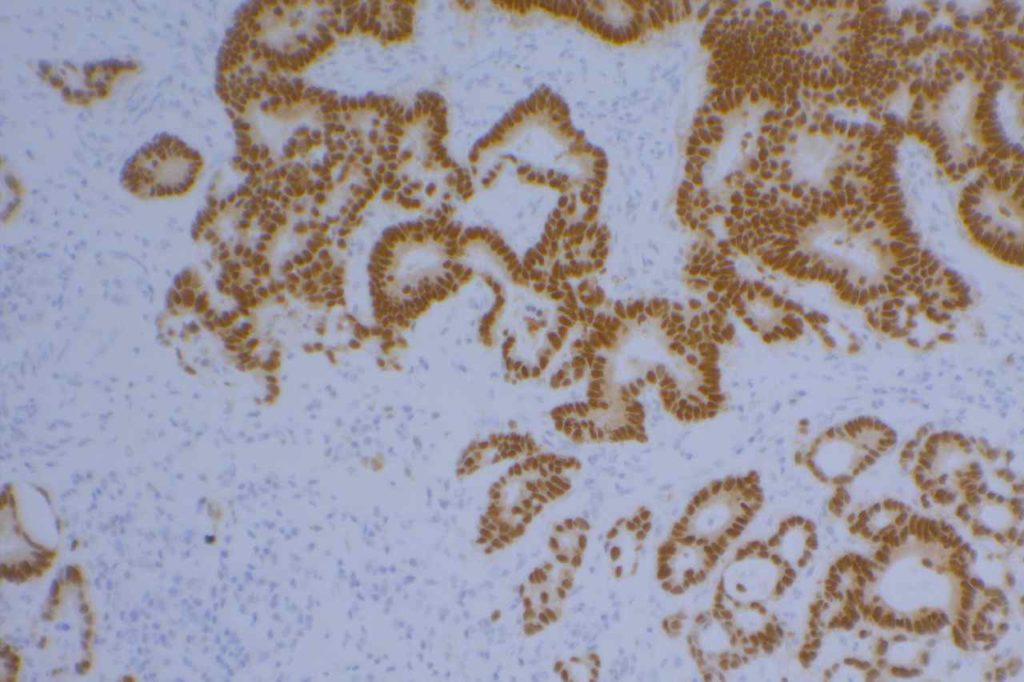
Photomicrograph
References
Werling, R. W., Yaziji, H., Bacchi, C. E., & Gown, A. M. (2003). CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. The American Journal of Surgical Pathology, 27(3), 303–310. Link: http://www.ncbi.nlm.nih.gov/pubmed/?term=American+Journal+of+Surgical+Pathology%2C+27(3)%2C+303%E2%80%93310.
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236 Link: http://www.ncbi.nlm.nih.gov/pubmed/?term=Markers+of+adenocarcinoma+characteristic+of+the+site+of+origin%3A+development+of+a+diagnostic+algorithm
Enriquez, M. L., Baloch, Z. W., Montone, K. T., Zhang, P. J., & LiVolsi, V. A. (2012). CDX2 expression in columnar cell variant of papillary thyroid carcinoma. American Journal of Clinical Pathology, 137(5), 722–726. doi:10.1309/AJCPXE3PUBWVZCGZ Link: http://www.ncbi.nlm.nih.gov/pubmed/22523209
Cameselle-Teijeiro, J., Alberte-Lista, L., Peteiro-Gonzalez, D., Abdulkader-Nallib, I., Reyes-Santias, R., Soares, P., et al. (2012). CDX2 Expression in Some Variants of Papillary Thyroid Carcinoma. American Journal of Clinical Pathology, 138(6), 907–910. doi:10.1309/AJCP1BGCA6MFCNKH Link: http://www.ncbi.nlm.nih.gov/pubmed/23161723
Borrisholt, M., Nielsen, S., & Vyberg, M. (2013). Demonstration of CDX2 is highly antibody dependant. Applied Immunohistochemistry & Molecular Morphology : AIMM / Official Publication of the Society for Applied Immunohistochemistry, 21(1), 64–72. doi:10.1097/PAI.0b013e318257f8aa Link: http://www.ncbi.nlm.nih.gov/pubmed/?term=Demonstration+of+CDX2+is+highly+antibody+dependant
Werling, R. W., Yaziji, H., Bacchi, C. E., & Gown, A. M. (2003). CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. The American Journal of Surgical Pathology, 27(3), 303–310. Link: http://www.ncbi.nlm.nih.gov/pubmed/?term=CDX2%2C+a+highly+sensitive+and+specific+marker+of+adenocarcinomas+of+intestinal+origin%3A+an+immunohistochemical+survey+of+476+primary+and+metastatic+carcinomas