CLL/SLL represents a B-cell neoplasm of small lymphocytes which involve a combination of peripheral blood, bone marrow, and/or lymph nodes. When peripheral blood predominates, then it is referred to as CLL, and when it presents as predominately nodal involvement it is referred to as SLL. This is the same disorder with different manifestations.
SLL criteria are lymphadenopathy without evidence of cytopenias secondary to bone marrow involvement by CLL/SLL and <5,000/uL peripheral blood monoclonal B-cell lymphocytosis.
Based on peripheral blood parameters, CLL is defined as a monoclonal B-cell lymphocyte count of >5,000/uL. In a case of monoclonal B-cells with characteristics and immunophenotype of CLL that fails to reach the diagnostic threshold, then it is referred to as “monoclonal B-cell lymphocytosis” (MBL). There may be a tissue-based analogous lesion to MBL where lymph nodes containing SLL without proliferation centers and are <1.5 cm in size behave in a very indolent manner.
CLL/SLL will often have a long (relative indolent) course that can last many years (>10 years), but is not considered “curable”. Richter syndrome will occur in 5-10% of cases where there will be transformation to a diffuse large B-cell lymphoma. CLL/SLL also results in immune system dysfunction (not well understood), but patients are more susceptible to infections. Autoimmune hemolytic anemia may occur in ~5% of cases, which can typically be successfully treated with steroid therapy.
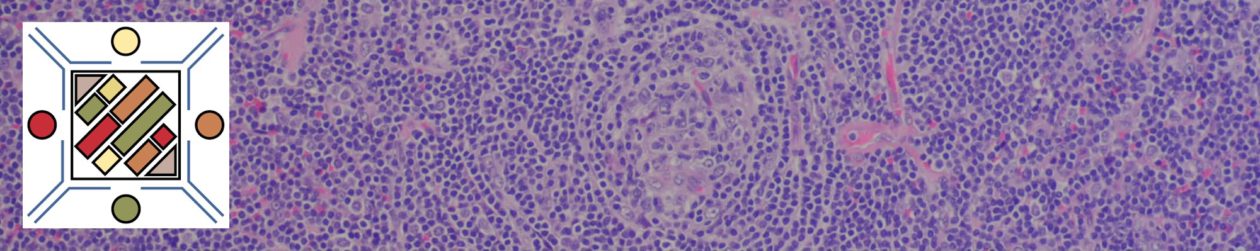
Morphology
Characteristic neoplastic B-cells are small and have round to mildly irregular nuclei (mantle cell lymphoma cells tend to be more irregular). The nuclear chromatin is dense with variable lightened areas giving the resemblence of a soccer ball or “snickerdoodle” cookie. Proliferation centers are characteristic of CLL/SLL and are composed of slightly larger lymphocytes with slightly more cytoplasm and contain mitotically active cells (sometimes express cyclin D1, but not diffuse expression as in mantle cell lymphoma).
Molecular
There are no characteristic translocations that define CLL/SLL (unlike many other lymphomas), but there are some genetic abnormalities that are commonly encountered and often carry prognostic significance. The most common genetic abnormalities include: deletion 13q14.3, deletion 11q, deletion 17p, and trisomy 12q. CLL/SLL appears to have to origins, either as a naive (unmated Ig) B-cells or as post-germinal center memory B-cells (mutated Ig). Unmutated IgHV (naive B-cells, >98% identity with the gremlin sequence) cases of CLL/SLL are associated with a more aggressive course and poorer prognosis. ZAP-70 and CD38 are used as surrogate markers for mutational status (analogous to the Hans’ algorithm in DLBCL). MYD88 mutation not typically found (2-3%).
Immunophenotype
CLL/SLL has a characteristic immunopheotype of CD19+ CD20+ (dim by flow cytometry), CD5+, and CD23+. The B-cell expression of CD20 and associated light chain restriction appears dimer by flow cytometry compared to mantle cell lymphoma.
|
Negative
|
|
|
Positive (92% by flow cytometry, 94% by IHC), often will have a dimmer pattern compared to CD3+ T-cells in the background.
|
|
|
Negative
|
|
|
Positive
|
|
|
Positive. Up to 20% of cases may lose expression at some point after rituximab therapy. (dim expression on flow cytometry)
|
|
|
Positive (94% by flow cytometry, 88% by IHC)
|
|
|
Often positive. CD43 is a T-cell markers, which may be aberrantly expressed in many different B-cell lymphomas. There is no specificity as to sub classification of B-cell neoplasms with CD43 alone.
|
|
|
Approximately 50-60% of CLL cases are ZAP-70 positive, which is associated with a shorter time to therapy (3-yr. risk 83% vs. 31% for ZAP-70 negative).
|
|
|
p53
|
p53 can be a surrogate marker for del(17p), which is an adverse prognostic indicator. p53 expression is very specific for del(17p), but the sensitivity is varied among the different studies (~30% in the largest). Therefore, if it is negative, it doesn’t help. Expression has been associated with poor response to chemotherapy and Alemtuzumab.
|
|
weak expression / may be negative. Better characterized by flow cytometry.
|
|
|
Some expression may be seen in proliferation centers.
|
|
|
Proliferation index of neoplastic cells is usually low as CLL/SLL is generally a low-grade lymphoma. A higher proliferation index may raise concern of a more aggressive disease course or different lymphoma such as mantle cell lymphoma.
|
|
|
Negative in CLL/SLL, although some expression may be detected in proliferation centers (up to 30%, these areas are negative for SOX11).
|
|
|
Expression identified in >95% of cases (including CD5- cases)
|
|
|
Negative
|
CD2 and CD33 have been noted to be aberrantly expressed in cases of CLL/SLL in approximately 17% of cases. [Dalton, et. al.]
Given that as many as 23% of mantle cell lymphoma cases may express CD23, regular IHC staining with cyclin D1 may be helpful to exclude occasional cases of mantle cell lymphoma that may mimic CLL/SLL. Proliferation centers in CLL/SLL may show cyclin D1 expression.
Adverse Prognostic Indicators
- Deletions 11q or 17p
- NOTCH1 mutations
- ZAP-70 expression (positive expression is surrogate marker for unmutated IgHV) can be performed by IHC (positive defined by at least weak 1+ expression in a majority of the tumor cells). ZAP-70 IHC can be performed on peripheral blood samples (Roullet, et. al)
- CD38 (by flow cytometry, may not be as reliable as other markers)
- Absence of somatic hypermutation
- Large/confluent and/or highly proliferative proliferation centers
- p53 expression by IHC has been associated with 17p (TP53) deletion, but routine use is controversial due to issues with reproducibility of published results.
2016 WHO revision
- Cytopenia and/or disease related symptoms are NOT sufficient to make the diagnosis of CLL without an absolute lymphocytosis >5,000/mL.
References
Robbins and Cotran Pathologic Basis of Disease. V Kumar, et al. 9th Edition. Elsevier Saunders. 2015. pp. 593-594.
Weissmann S, Roller A, Jeromin S, Hernández M, Abáigar M, Hernández-Rivas JM, et al. Prognostic impact and landscape of NOTCH1 mutations in chronic lymphocytic leukemia (CLL): a study on 852 patients. Leukemia. 2013;27: 2393–2396. doi:10.1038/leu.2013.218
Montgomery ND, Mathews SP. Transformation in Low-grade B-cell Neoplasms. Surg Pathol Clin. 2016;9: 79–92. doi:10.1016/j.path.2015.09.004
Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21: 116–131. doi:10.1097/PAI.0b013e31825d550a
Mauro FR, Foa R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95: 2786–2792.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127: 2375–2390. doi:10.1182/blood-2016-01-643569
Foster AE, Nguyen TT, Al-Hammadi N, Frater JL, Hassan A, Kreisel F. Clinical Presentation, Progression, and Outcome of Patients With Clonal B-Cell Counts of Less Than 5 x 109/L, 5 to 10 x 109/L, and More Than 10 x 109/L and Chronic Lymphocytic Leukemia Immunophenotype. Am J Clin Pathol. 2014;143: 70–77. doi:10.1309/AJCPIXUB5MZK8ECI
Gradowski JF, Sargent RL, Craig FE, Cieply K, Fuhrer K, Sherer C, et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma With Cyclin D1 Positive Proliferation Centers Do Not Have CCND1 Translocations or Gains and Lack SOX11 Expression. Am J Clin Pathol. 2012;138: 132–139. doi:10.1309/AJCPIVKZRMPF93ET
Admirand JH, Knoblock RJ, Coombes KR, Tam C, Schlette EJ, Wierda WG, et al. Immunohistochemical detection of ZAP70 in chronic lymphocytic leukemia predicts immunoglobulin heavy chain gene mutation status and time to progression. Mod Pathol. 2010;23: 1518–1523. doi:10.1038/modpathol.2010.131
Cook JR. Nodal and leukemic small B-cell neoplasms. Mod Pathol. 2013;26 Suppl 1: S15–28. doi:10.1038/modpathol.2012.180
Dalton, et. al. “Small Lymphocytic Lymphoma.” Pathology Case Reviews. Vol. 9, No. 5, September/October 2004.
Chang H, Jiang AM, Qi CXY. Aberrant nuclear p53 expression predicts hemizygous 17p (TP53) deletion in chronic lymphocytic leukemia. Am J Clin Pathol. 2010;133: 70–74. doi:10.1309/AJCPEPX1C7HHFELK
Marinelli M, Raponi S, Giudice ID, de Propris MS, Nanni M, Intoppa S, et al. Is the Aberrant Expression of p53 by Immunocytochemistry a Surrogate Marker of TP53 Mutation and/or Deletion in Chronic Lymphocytic Leukemia? Am J Clin Pathol. 2011;135: 173–174. doi:10.1309/AJCPFMV43IFPRTWK
Roullet M, Sargent R, Pasha T, Cajiao I, Elstrom R, Smith T, et al. ZAP70 expression assessed by immunohistochemistry on peripheral blood: a simple prognostic assay for patients with chronic lymphocytic leukemia. Appl Immunohistochem Mol Morphol. 2007;15: 471–476. doi:10.1097/01.pai.0000213152.41440.34
Gibson SE, Swerdlow SH, Ferry JA, Surti U, Dal Cin P, Harris NL, et al. Reassessment of small lymphocytic lymphoma in the era of monoclonal B-cell lymphocytosis. Haematologica. 2011;96: 1144–1152. doi:10.3324/haematol.2011.042333