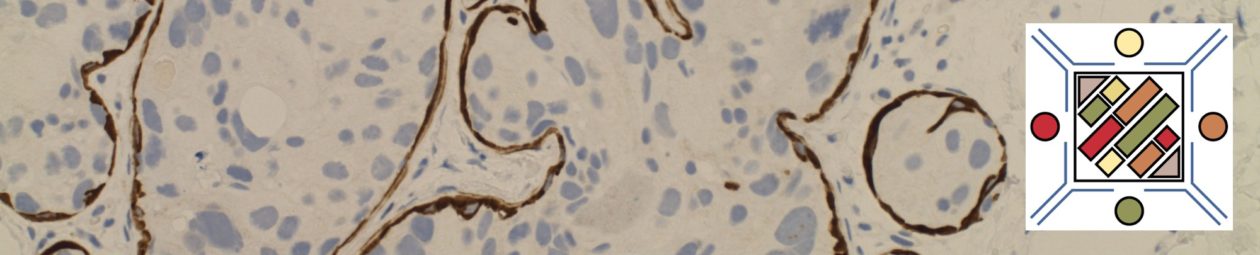
IHC stain expression pattern for various IHC antibodies in breast carcinoma.
|
IHC Stain
|
Comments
|
|
|
50-70%
|
|
|
30-60%
|
|
|
>80%
|
|
|
<10%
|
|
|
10-25%
|
|
|
0%
|
|
|
0%
|
|
|
0%
|
|
Mesothelin
|
<10%
|
Clin Cancer Rs 2005;11(10) May 15, 2005.
When evaluating for a possible breast primary, it is usually part of a larger differential diagnosis. CK7 and CK20 is the common starting point for carcinomas of unknown primary site (CUPS), and breast has a characteristic CK7+/CK20= profile. Unfortunately, this pattern is not uncommon, and more specific markers need to be performed. Two specific breast markers are GCDFP-15 and ER., but their sensitivity while good is limited. GATA-3 is a relatively newer antibody, which shows excellent sensitivity with good specificity, and should be strongly considered to be part of an antibody panel in work-up of potential breast carcinoma cases.
- GCDFP-15 is very specific for breast carcinoma in the setting of CUPS, but limited sensitivity.
- ER expression may be highly suggestive of a breast primary (especially epidemiologically), but it is not as specific.
- Other female organs (ovary/uterus) not uncommonly express ER, and practically any tissue can occasionally excess ER.
- GATA-3 appears to have excellent sensitivity (>90%) with good (not perfect) specificity.
Liu, et al (Biocare Medical, Concord, CA)
|
Tumor
|
GATA-3
|
GCDFP-15
|
MGB
|
|
Breast Carcinoma
|
94%
|
35-55%
|
65-70%
|
|
ER-negative breast ca.
|
69%
|
15%
|
35%
|
|
Urothelial Carcinoma
|
86%
|
|
|
Breast Metastasis vs. Ovarian Ca. Primary
|
Tumor
|
WT-1
|
CA-125
|
GCDFP-15
|
|
Primary Ovarian Ca.
(N=41)
|
76%
|
73%
|
0%
|
|
Metastatic Breast Ca.
(N-40)
|
3%
|
10%
|
43%
|
|
P-Value
|
<0.001
|
<0.001
|
<0.001
|
Chen, USCAP, 2004
References
Liu, H., Shi, J., Wilkerson, M. L., & Lin, F. (2012). Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. American Journal of Clinical Pathology, 138(1), 57–64. doi:10.1309/AJCP5UAFMSA9ZQBZ
Miettinen, M., McCue, P. A., Sarlomo-Rikala, M., Rys, J., Czapiewski, P., Wazny, K., et al. (2014). GATA3: A Multispecific But Potentially Useful Marker in Surgical Pathology: A Systematic Analysis of 2500 Epithelial and Nonepithelial Tumors. The American Journal of Surgical Pathology, 38(1), 13–22. doi:10.1097/PAS.0b013e3182a0218f
Clin Cancer Rs 2005;11(10) May 15, 2005.
Chen, USCAP, 2004