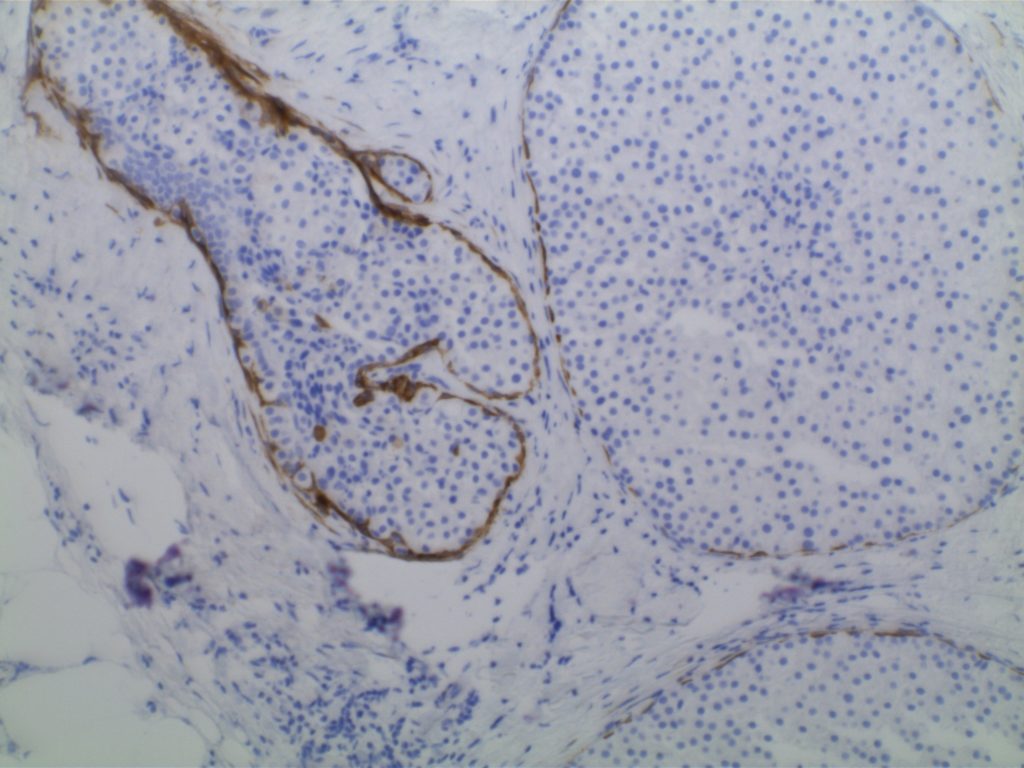
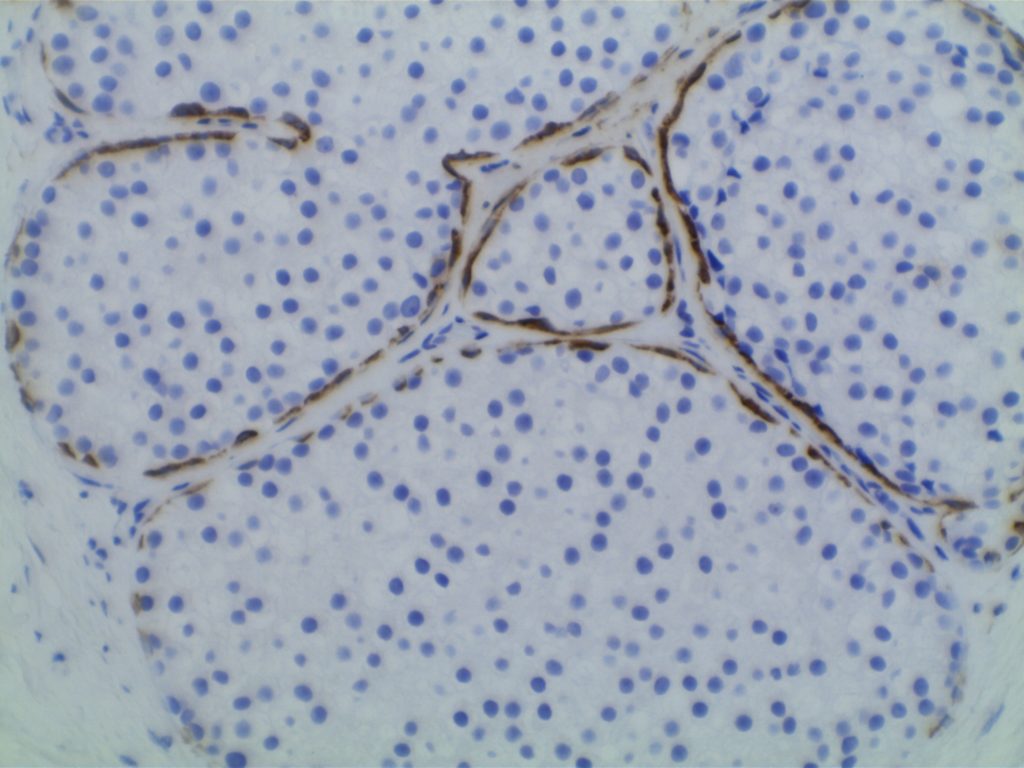
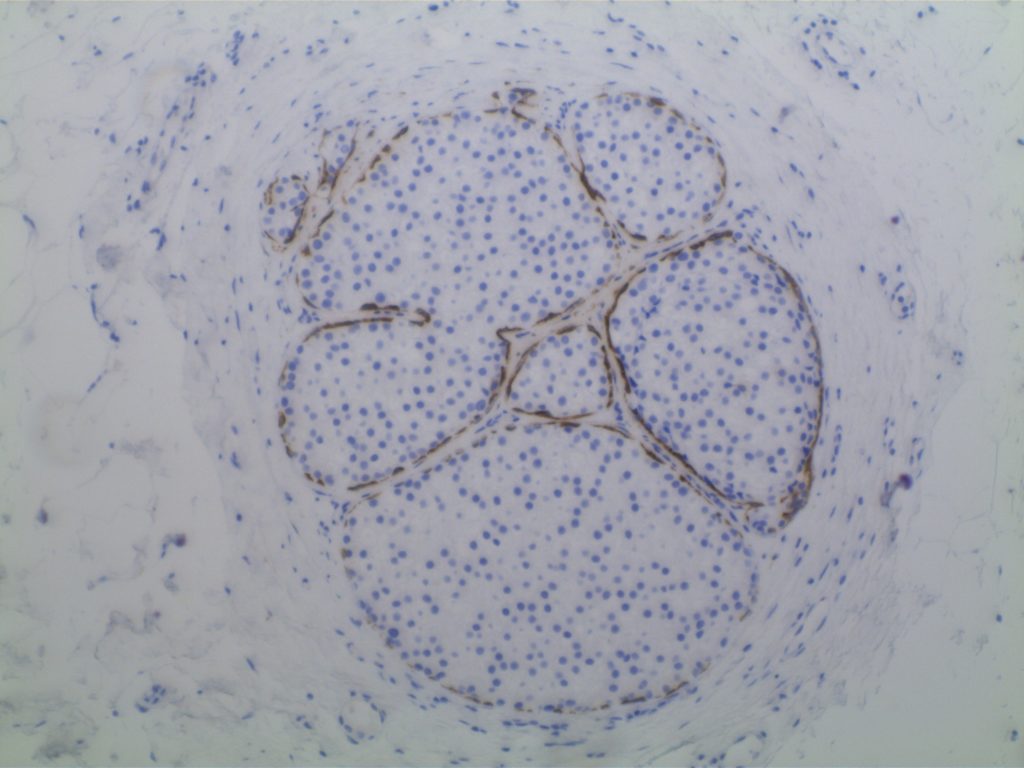
Lobular vs. ductal differentiation in breast lesions can on occasion be problematic. E-cadherin is well described as a marker of ductal differentiation, but aberrant expression has be reported in 2-16% of cases of lobular neoplasia. Some authors suggest not changing the interpretation/diagnosis based on expression of E-cadherin is the morphology is consistent with lobular carcinoma. This author is not sure why one would do the stain to begin with if the morphologic characteristics are consistent with lobular carcinoma. 34betaE12 (CK903) has been described as a sensitive and specific in showing perinuclear dot-like expression in cases of lobular carcinoma and negativity in ductal carcinomas. The combined panel of 34betaE12 and E-cadherin makes a nice complimentary panel to evaluate between lobular and ductal differentiation in breast carcinomas when morphology alone is not clear.
|
IHC Stain
|
DCIS
|
LCIS
|
|
E-Cadherin
|
+
|
=
|
|
34betaE12
|
=
|
+
|
References
Liu H. Application of immunohistochemistry in breast pathology: a review and update. Arch Pathol Lab Med. 2014;138(12):1629–1642. doi:10.5858/arpa.2014-0094-RA.
Bratthauer GL, Moinfar F, Stamatakos MD, et al. Combined E-cadherin and high molecular weight cytokeratin immunoprofile differentiates lobular, ductal, and hybrid mammary intraepithelial neoplasias. Hum Pathol. 2002;33(6): 620–627.