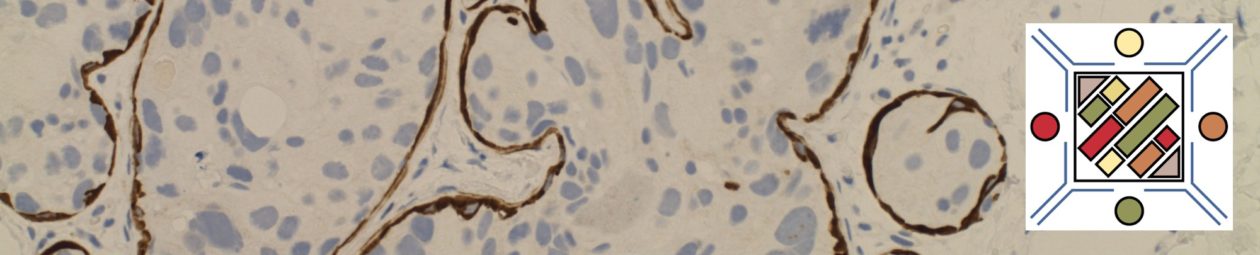
Follicular Lymphoma (FL) is a mature B-cell lymphoma, which recapitulates or resembles germinal center B-cells. Most cases (~85%) harbor the characteristic t(14;18), which juxtaposes the BCL-2 gene on chromosome 18 with the IgH gene on chromosome 14 (and hence BCL-2 IHC protein expression). Most patients (~80-85) will present with advanced disease (stage III/IV), and bone marrow involvement is found in ~40% of cases with characteristic paratrabecular aggregates (mantle cell lymphoma and lymphoplasmacytic lymphoma may also have paratrabecular lymphoid aggregates). Most of the cases that lack the t(14;18) IgH/BCL-2 translocation (and are BCL-2 negative) are typically grade 3 FLs with a BCL-6 translocation (~10-15%). BCL-6 translocations can be evaluated for by FISH analysis, but the finding is NOT specific for FL.
Over time 30-50% of cases transform to diffuse large B-cell lymphoma (DLBCL). In a small subset of transformations, a second “hit” with a MYC translocation will occur resulting in a very aggressive high grade large B-cell lymphoma: the so-called “double hit” lymphoma.
Morphology
FL usually has at least a component of nodularity (+/- diffuse areas). There are two cell types that make up FL, centrocytes and centroblasts. Centrocytes are small cleaved cells with folded irregular nuclei. Centorblasts are large cells with more open chromatin, multiple nucleoli, and more cytoplasm compared to centrocytes.
Sometimes FL can have patterns that resemble marginal zone lymphoma, and can even have plasmacytic differentiation. Therefore, it is important that a panel of markers be used to identify (or exclude) evidence of germinal center differentiation. Occasional cases can have Hodgkin-like cells.
Immunophenotype
|
Marker
|
Comment
|
|
Negative
|
|
|
Positive
|
|
|
Positive
|
|
|
Positive
|
|
|
Positive (~90%), negative cases do not contain the t(14;18), which is more common in grade 3 cases
|
|
|
Positive, (~88%)
|
|
|
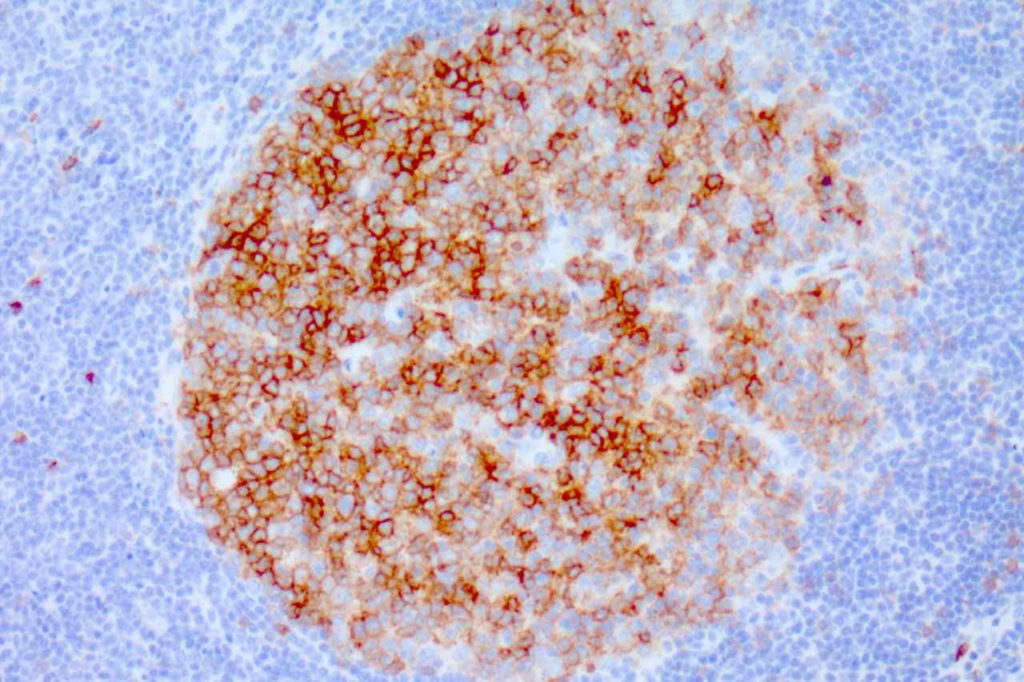
CD35
|
Highlights the follicular dendritic meshwork associated with FL.
|
|
Usually negative, higher grade lesions may be positive
|
|
|
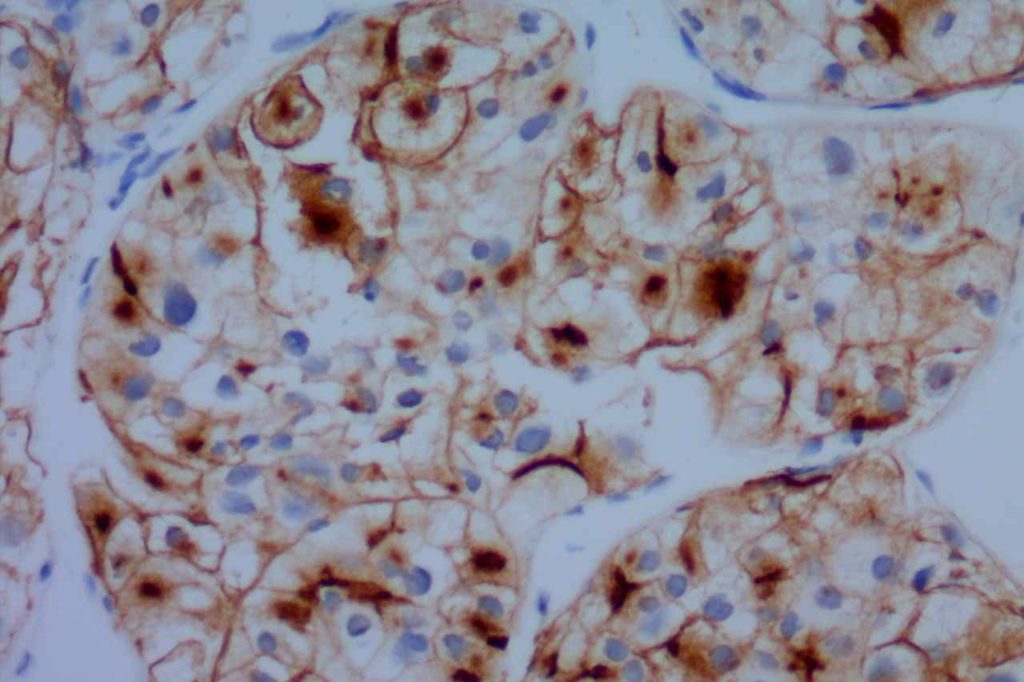
Variable, shows low expression in low-grade processes, in distinct contrast to the high proliferation index and polarity associated with reactive germinal centers.
|
|
|
Negative
|
|
|
|
FL is typically expresses CD19, CD20, CD10, Bcl-6, and BCL-2 (~90%). CD5 is not expressed in FL.
- Normal reactive germinal centers do not express Bcl-2. In 90% of cases of FL, bcl-2 is expressed, which serves as a diagnostic tissue marker in lymphoma sections.
- CD23 expression by flow cytometry has been associated with lower grade FLs (e.g. grade 1 & 2) and better survival.
Grading
- Grade 1 & 2: <= 15 centroblasts/HPF (based on 0.159 mm² HPF)
- Grade 3: > 15 centroblasts/HPF (based on 0.159 mm² HPF)
- 3A: Centrocytes present in the background
- 3B: NO centrocytes present in the background (not associated with the IgH/BCL-2 rearrangement, and usually lacks expression of CD10 and BCL-2; often MUM-1+)
Grade 1 & 2 behave in a similar fashion as a low grade lymphoma. Grade 3 FL behaves as an intermediate grade lymphoma. Grading of FL with counting of large cells must take into consideration the field diameter of the microscope being used. The counts above are based on a F.N. 18 (0.159 mm² @ 40X). Most convention pathology scopes today are F.N. 22 (0.247 mm² @ 40X), and adjustments are necessary.
Pattern
- Predominately follicular: >75% follicular/nodular architecture
- Follicular and diffuse: 25-75% Diffuse areas or follicular/nodular architecture
- Preominately diffuse: <25% follicular/nodular areas (diffuse areas of otherwise grade 3 FL, then that component should be described as a separate component of diffuse large B-cell lymphoma)
Special Subtypes
- Large B-Cell Lymphoma with IRF4 Rearrangement
- Pediatric Follicular Lymphoma
- Occurs in children and young adults with an excellent prognosis, marked male predilection
- The morphology is high-grade (FL grade 3) appearing
- BCL-2 negative, lacK t(14;18)
- CD10 + (usually)
- MUM-1 negative
- Associated with TNFRSF14 deletions of mutations
- Localized process, usually in the head and neck area
- Duodenal Follicular Lymphoma
- Localized lesion
- Grade 1-2 pattern
- CD10/BCL-2 +
- t(14;18) present
- Lacks follicular dendritic meshwork
- Ki-67, low expression
- Excellent prognosis
- Predominately Diffuse Follicular Lymphoma with 1p36 deletion
- Localized mass (often inguinal)
- Diffuse pattern, grade 1/2
- Excellent prognosis
- Immunophenotype: CD20+, CD10+, BCL-2+, BCL-6+, CD23+ (subset of cases)
- t(14;18) NOT present
- 1p36 deletion (not specific)
- Lacks Bcl-2 rearrangement
- Primary Cutaneous Follicular Lymphoma
- In Situ Follicular Neoplasm (ISFN)
References
Robbins and Cotran Pathologic Basis of Disease. V Kumar, et al. 9th Edition. Elsevier Saunders. 2015. pp. 594-595.
Fedoriw Y, Dogan A. The Expanding Spectrum of Follicular Lymphoma. Surg Pathol Clin. 2016;9: 29–40. doi:10.1016/j.path.2015.11.001
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127: 2375–2390. doi:10.1182/blood-2016-01-643569
Xerri L, Dirnhofer S, Quintanilla-Martinez L, Sander B, Chan JKC, Campo E, et al. The heterogeneity of follicular lymphomas: from early development to transformation. Virchows Arch. 2016;468: 127–139. doi:10.1007/s00428-015-1864-y
MD DY-PW, BacSc F. A case of t (14; 18)-negative follicular lymphoma with atypical immunophenotype: usefulness of immunoarchitecture of Ki67, CD79a and follicular dendritic cell …. … Malaysian journal of …. 2014.
Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21: 116–131. doi:10.1097/PAI.0b013e31825d550a
Cook JR. Nodal and leukemic small B-cell neoplasms. Mod Pathol. 2013;26 Suppl 1: S15–28. doi:10.1038/modpathol.2012.180
Olteanu H, Fenske TS, Harrington AM, Szabo A, He P, Kroft SH. CD23 Expression in Follicular Lymphoma: Clinicopathologic Correlations. Am J Clin Pathol. 2011;135: 46–53. doi:10.1309/AJCP27YWLIQRAJPW
Gradowski JF, Jaffe ES, Warnke RA, Pittaluga S, Surti U, Gole LA, et al. Follicular lymphomas with plasmacytic differentiation include two subtypes. Mod Pathol. 2010;23: 71–79. doi:10.1038/modpathol.2009.146
Katzenberger T, Kalla J, Leich E, Stöcklein H, Hartmann E, Barnickel S, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113: 1053–1061. doi:10.1182/blood-2008-07-168682
Bayerl MG, Bentley G, Bellan C, Leoncini L, Ehmann WC, Palutke M. Lacunar and reed-sternberg-like cells in follicular lymphomas are clonally related to the centrocytic and centroblastic cells as demonstrated by laser capture microdissection. Am J Clin Pathol. 2004;122: 858–864. doi:10.1309/PMR8-6PHK-K4J3-RUH3