Lymphoid enhancer-binding factor 1 (LEF1) is a mediator of the Wnt/β-catenin pathway, which regulates cell proliferation and survival, and shows nuclear expression by immunohistochemistry. It is normally expressed in T cells and pre-B cells. Mature B cells do not express LEF1.
Tag Archives: CLL/SLL
B-PLL
B-Cell Prolymphocytic Leukemia
A rare mature B-cell leukemia (~1% of cases) composed of medium sized lymphoid cells (2x size of normal lymphocyte), which typically has splenic and bone marrow involvement.
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)
CLL/SLL represents a B-cell neoplasm of small lymphocytes which involve a combination of peripheral blood, bone marrow, and/or lymph nodes. When peripheral blood predominates, then it is referred to as CLL, and when it presents as predominately nodal involvement it is referred to as SLL. This is the same disorder with different manifestations.
Continue reading Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)
Monoclonal B-Cell Lymphocytosis (MBL)
Monoclonal B-cell lymphocytosis (MBL) was defined by the International Familial CLL consortium in 2005 as a monoclonal B-cell lymphocyte population in the peripheral blood <5,000/uL without evidence of lymphadenopathy (i.e. SLL), an autoimmune/infectious disease or other features diagnostic of a B-cell lymphoproliferative disorder. The 2016 WHO hematopathology revision dropped the requirement of cytopenias or disease related symptoms as adequate to make the diagnosis of CLL.
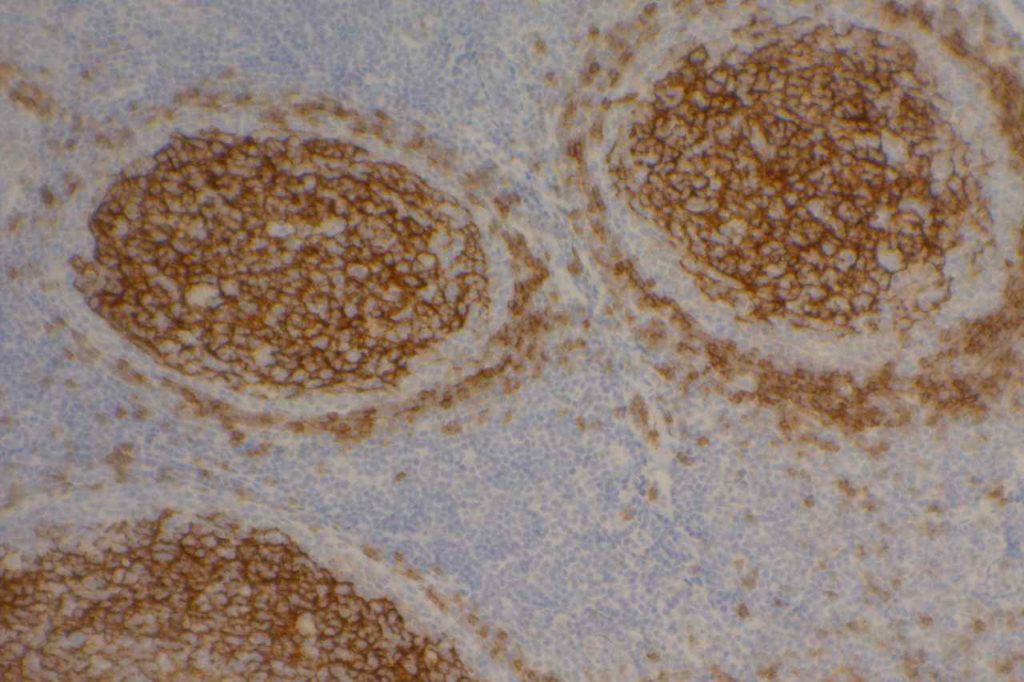
CD23
CD23 is a transmembrane glycoprotein expressed by different hematopoietic cells and is a low-affinity receptor for IgE. It is also involved in promoting survival of B-cells in the germinal center. CD23 is useful as a follicular cell dendritic cell marker and is classically expressed in cases of CLL/SLL. CD23 has been identified in many types of lymphomas, but is most commonly used to differentiate between CLL/SLL (CD23+) and mantle cell lymphoma (CD23-). This testing is typically performed by flow cytometry, but immunohistochemisty for CD23 is available. Expression of CD23 has been associated with better prognosis (at least in limited published data) in follicular lymphoma, CLL/SLL, mantle cell lymphoma, and diffuse large B-cell lymphoma dependent upon expression characteristics. CD23 is not commonly performed/used as a prognostic marker for B-cell lymphomas.
Rarely CD23 may be expressed in cases of Hairy cell leukemia (17%) and DLBCL (16%). Approximately 70% of Mediastinal large B-cell lymphoma cases express CD23. Practically, this IHC marker is used as a follicular dendritic cell marker and to help differentiate CLL/SLL from mantle cell lymphoma. Follicular dendritic cell tumors will also express CD23 like CD21. CD21 is more sensitive compared to CD23 as a follicular dendritic marker.
Follicular Lymphoma (FL) – CD23 has been found to be expressed in some cases of FL, especially from inguinal lymph nodes, and prognosis appears comparatively better. Olteanuet. al found that 87% of inguinal lymph nodes expressed CD23, compared to 61% from other sites, and that survival was prolonged more in CD23+ cases.
Diffuse Large B-Cell Lymphoma – A subset of DLBCLs may express CD23, which may have a better prognosis (CD23 is not commonly performed for this purpose).
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) – CD23 expression is characteristic of CLL/SLL, particularly in comparison to another CD5+ lymphoma, mantle cell lymphoma. Strong membrane expression has been associated with a better outcome. DiRaimondo,et. al found ~6% of CLL cases to be CD23 negative (flow cytometry), and they had a worse prognosis. Many of these cases may have been misdiagnosed mantle cell lymphomas.
Mantle Cell Lymphoma (MCL) – CD23 is characteristically negative in MCL, which helps to differentiate it from CLL/SLL. However, ~21% of cases of MCL were found to be CD23+ by Gao,et. al, and other studies have shown CD23 expression in MCL ranging from 0% to 45% (most data appears to be based on flow cytometry).
CD23 Expression Pattern
- CLL/SLL – characteristically expressed (6% may be negative, probably much lower)
- Mantle cell lymphoma may be CD23+ (21%+, 0-45%)
- B-cell Lymphomas (e.g. some DLBCL and follicular lymphomas may show expression)
- Follicular Dendritc Cells (not as sensitive as CD21)
- B-cells in mantle zone of lymphoid follicles
Photomicrographs
Reference
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 69.
Linderoth J, Jerkeman M, Cavallin-Stahl E, et al. Immunohistochemical expression of CD23 and CD40 may identify prognostically favorable subgroups of diffuse largeB-cell lymphoma: a Nordic Lymphoma Group Study.ClinCancer Res.2003;9:722-728.
Olteanu H, Fenske TS, Harrington AM, Szabo A, He P, Kroft SH. CD23 Expression in Follicular Lymphoma: Clinicopathologic Correlations. Am J Clin Pathol. 2011;135: 46–53. doi:10.1309/AJCP27YWLIQRAJPW
Gao J, Peterson L, Nelson B, Goolsby C, Chen Y-H. Immunophenotypic variations in mantle cell lymphoma. Am J Clin Pathol. 2009;132: 699–706. doi:10.1309/AJCPV8LN5ENMZOVY
Troxell ML, Schwartz EJ, van de Rijn M, Ross DT, Warnke RA, Higgins JP, et al. Follicular dendritic cell immunohistochemical markers in angioimmunoblastic T-cell lymphoma. Appl Immunohistochem Mol Morphol. 2005;13: 297–303.
Dalton RR, Admirand JH, Medeiros LJ. Small Lymphocytic Lymphoma. Pathology Case Reviews. 2004;9: 7.
DiRaimondo F, Albitar M, Huh Y, O’Brien S, Montillo M, Tedeschi A, et al. The clinical and diagnostic relevance of CD23 expression in the chronic lymphoproliferative disease. Cancer. 2002;94: 1721–1730. doi:10.1002/cncr.10401
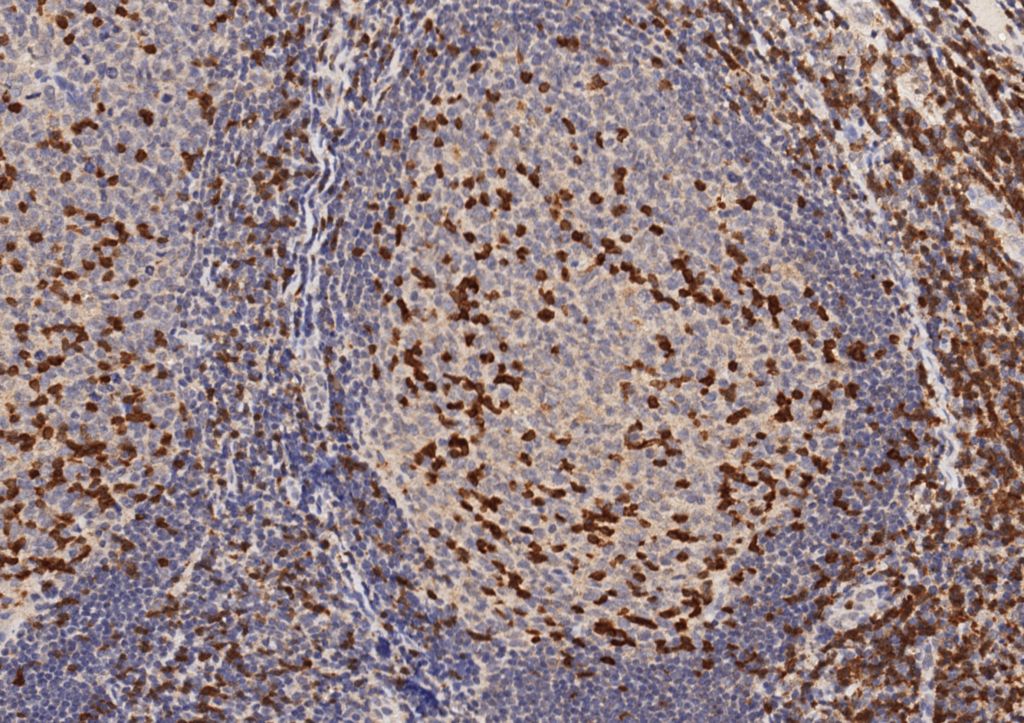
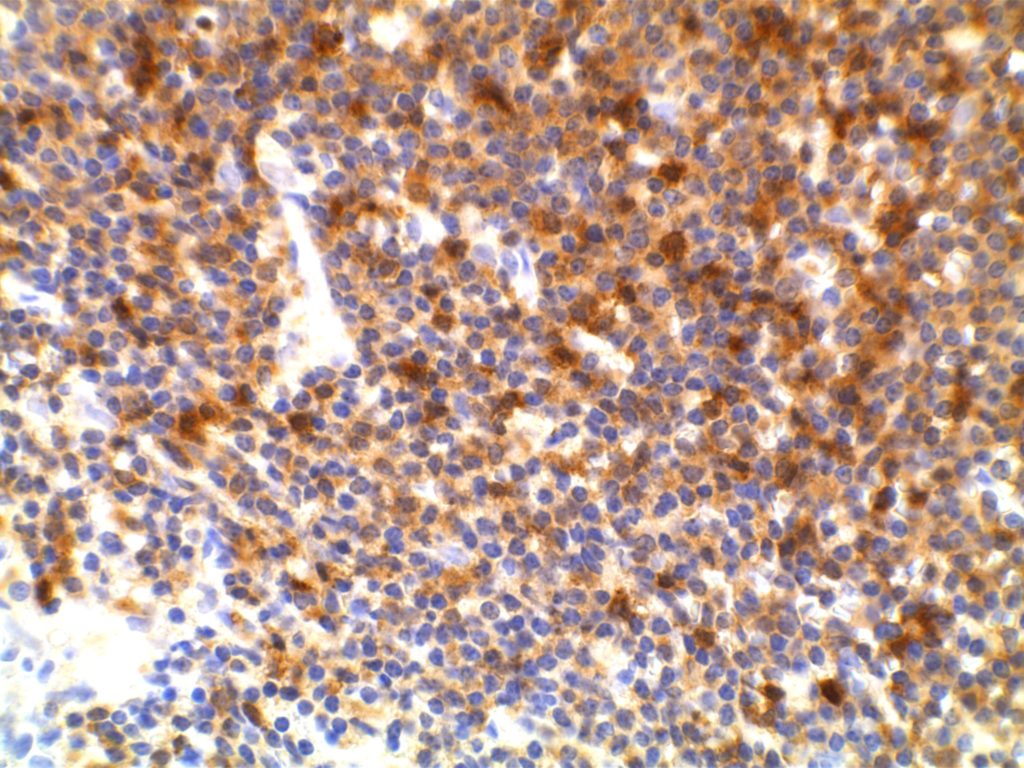
ZAP-70
General
ZAP-70 (zeta-associated protein-70) is a surrogate marker for the somatic mutation status of immunoglobulin heavy chain (IGHV) in CLL. Unfortunately, attempts to utilize flow cytometry for this purpose has resulted in unreliable results. ZAP-70 expression by IHC has been shown to have an increased risk of progression to therapy requirement (3-yr risk 83% vs. 31% for ZAP-70 negative) [Modern Pathology (2010)23,1518-1523]. ZAP-70 expression is not specific to CLL, and is not particularly useful for tumor sub-classification/prognosis outside the setting of CLL.
ZAP-70 expression in B-cell lymphoid neoplasms (Carreras, J, et al).
|
Lymphoid Disorder
|
No.
|
ZAP-70 + (%)
|
|
Lymphoblastic Lymphoma
|
7
|
28%
|
|
Chronic Lymphocytic Leukemia
|
52
|
65%
|
|
Mantle Cell Lymphoma
|
36
|
8%
|
|
Classical
|
28
|
11%
|
|
Blastoid
|
8
|
0%
|
|
Follicular Lymphoma
|
19
|
0%
|
|
Marginal Zone Lymphoma
|
23
|
4%
|
|
MALT
|
11
|
0%
|
|
Nodal
|
5
|
20%
|
|
Splenic
|
7
|
0%
|
|
Diffuse Large B-Cell Lymphoma
|
45
|
2%
|
|
Burkitt Lymphoma
|
29
|
31%
|
|
Hodgkin Lymphoma
|
14
|
0%
|
Stain Interpretation
ZAP-70 is interpreted as negative or positive. The minimum positive expression is weakly positive( 1+) staining defined as granular cytoplasmic staining with nuclear blush in a majority of tumor cells. Strong positivity (2+) is defined as strong expression in a majority of tumor cells.
ZAP-70 will also stain T-cells in the background. Therefore, ZAP-70 should be interpreted with the accompaniment of CD3 and CD20, so that there is clear discernment between tumor and background lymphoid cells.
IHC on Peripheral Blood
One of the big problems to identify ZAP-70 expression in CLL is the material available for evaluation. Most material is based on peripheral blood, and flow cytometry has been difficult to analyze reliably for ZAP-70 expression. An alternative is to perform PERIPHERAL BLOOD MONONUCLEAR CELL (PBMC) PURIFICATION AND CELL BLOCK PREPARATION as described by Roullet, et. al. in which a cell block is prepared from peripheral blood on which IHC for ZAP-70 can be reliably performed. Please review Roullet’s article for complete technical details.
Photomicrographs
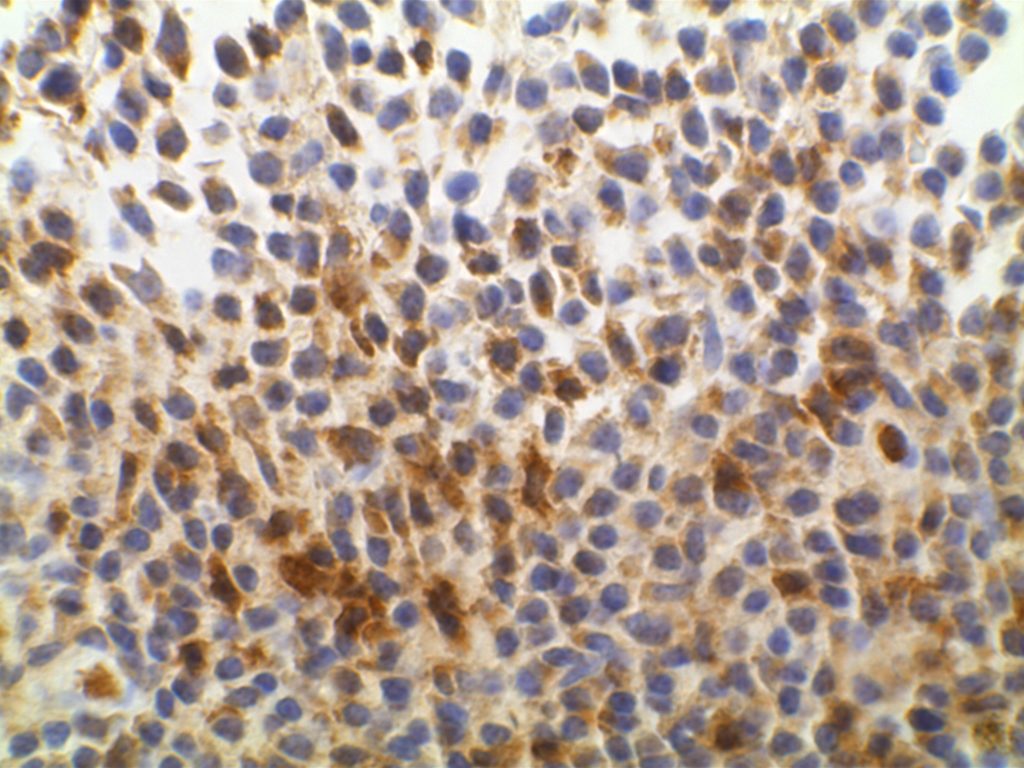
References
Admirand, J. H., Knoblock, R. J., Coombes, K. R., Tam, C., Schlette, E. J., Wierda, W. G., et al. (2010). Immunohistochemical detection of ZAP70 in chronic lymphocytic leukemia predicts immunoglobulin heavy chain gene mutation status and time to progression. Modern Pathology : an Official Journal of the United States and Canadian Academy of Pathology, Inc, 23(11), 1518–1523. doi:10.1038/modpathol.2010.131
Carreras, J., Villamor, N., Colomo, L., Moreno, C., Ramón y Cajal, S., Crespo, M., et al. (2005). Immunohistochemical analysis of ZAP-70 expression in B-cell lymphoid neoplasms. The Journal of Pathology, 205(4), 507–513. doi:10.1002/path.1727
Roullet, M., Sargent, R., Pasha, T., Cajiao, I., Elstrom, R., Smith, T., et al. (2007). ZAP70 expression assessed by immunohistochemistry on peripheral blood: a simple prognostic assay for patients with chronic lymphocytic leukemia. Applied Immunohistochemistry & Molecular Morphology : AIMM / Official Publication of the Society for Applied Immunohistochemistry, 15(4), 471–476. doi:10.1097/01.pai.0000213152.41440.34
Crespo, M., Bosch, F., Villamor, N., Bellosillo, B., Colomer, D., Rozman, M., et al. (2003). ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. The New England Journal of Medicine, 348(18), 1764–1775. doi:10.1056/NEJMoa023143
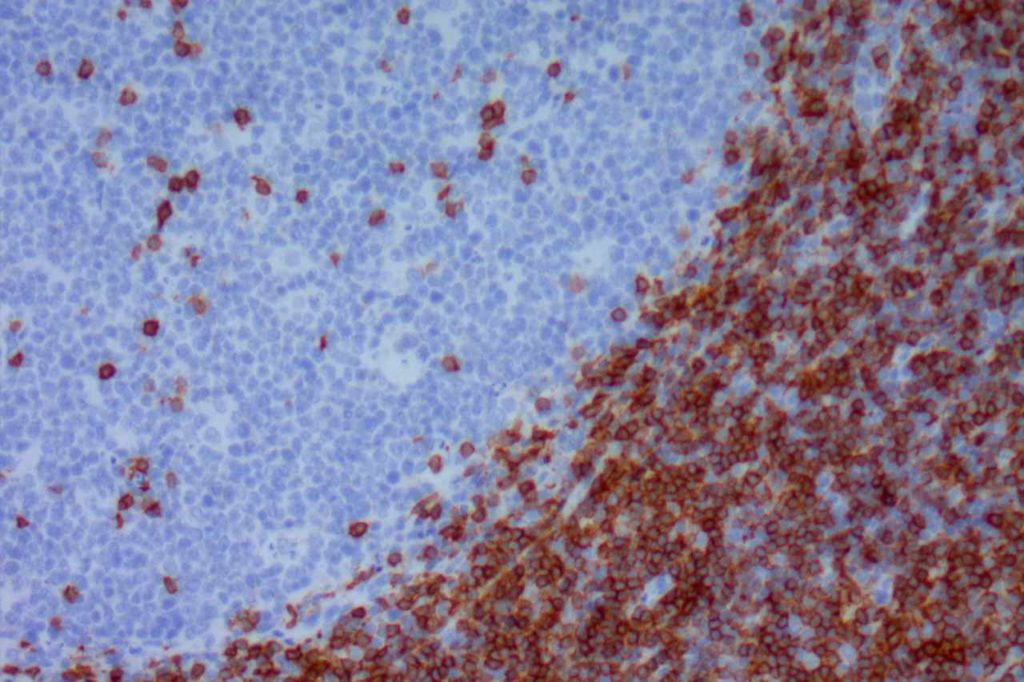
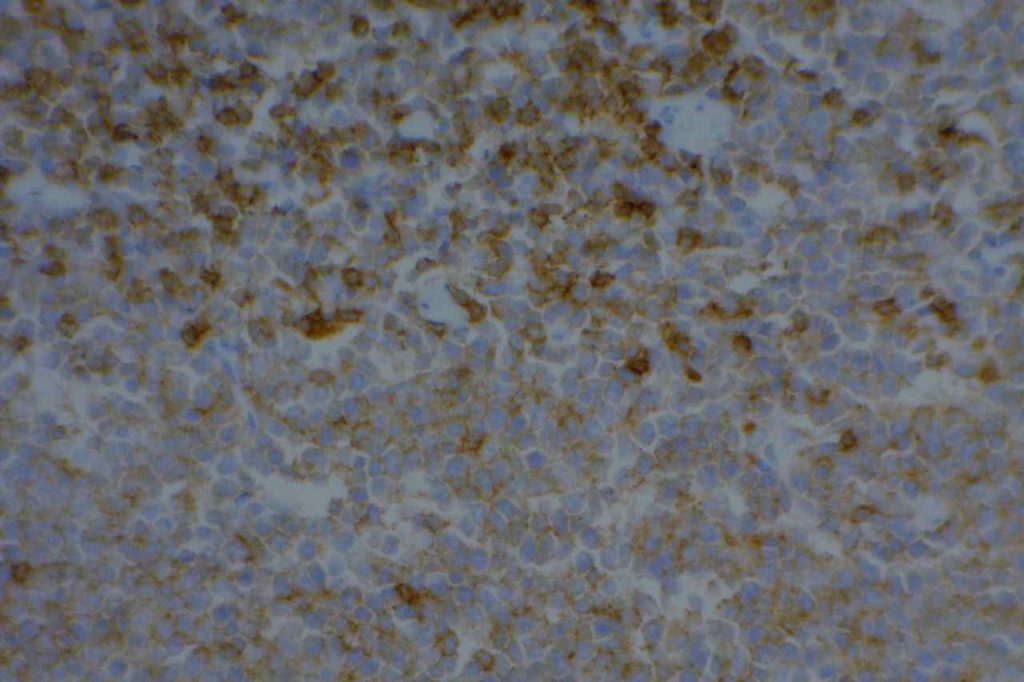
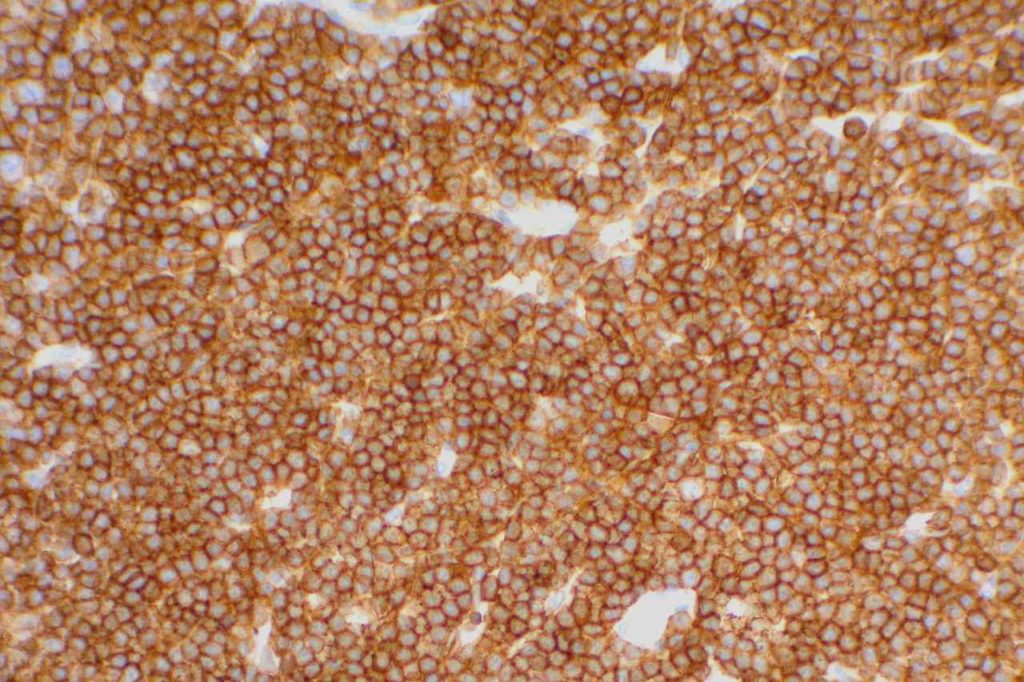
CD5
CD5 is a T-cell marker, although it is the least specific of the group (CD2, CD3, CD4, CD5, CD7, and CD8). CD5 co-expression on B-cells is characteristic of certain lymphomas, specifically CLL/SLL and Mantle Cell Lymphoma. A subset of DLBCLs may also express CD5, although there may be a question as to if that represents de novo lymphoma vs. “transformed” CLL/SLL or Mantle Cell Lymphoma.
The expression level of CD5 will often be less than the expression on background T-cells in cases of CLL/SLL and Mantle Cell Lymphoma, and this expression differentiation may be helpful by both IHC and flow cytometry.
CD5 Expression
- CLL/SLL (often dimmer than background T-cells) – Typically co-express CD5 & CD23. A small portion of cases may be CD5-. LEF1 is a newer marker that is expressed in CLL/SLL and may be helpful for confirming atypical cases of CLL/SLL.
- Mantle Cell Lymphoma – Approximately 12% may not express CD5. SOX11, Cyclin D1 (bcl-1) and FISH for t(11;14) are helpful in atypical cases.
- Nodal Marginal Zone Lymphoma – Up to 8-9% of cases may have co-expression of CD5
- Normal T-cells
- Diffuse Large B-cell Lymphoma (small subset, ~5%) – Associated with more aggressive course, bone marrow involvement, and CNS involvement.
- Lymphoplasmacytic Lymphoma (~5%)
- T-cell Lymphomas
Many different B and T-cell lymphomas have reported CD5, and while it is a useful marker, specificity should be determined with a broader set of markers in addition to the morphology of the lymphoma. Molecular studies may also be helpful.
Photomicrographs
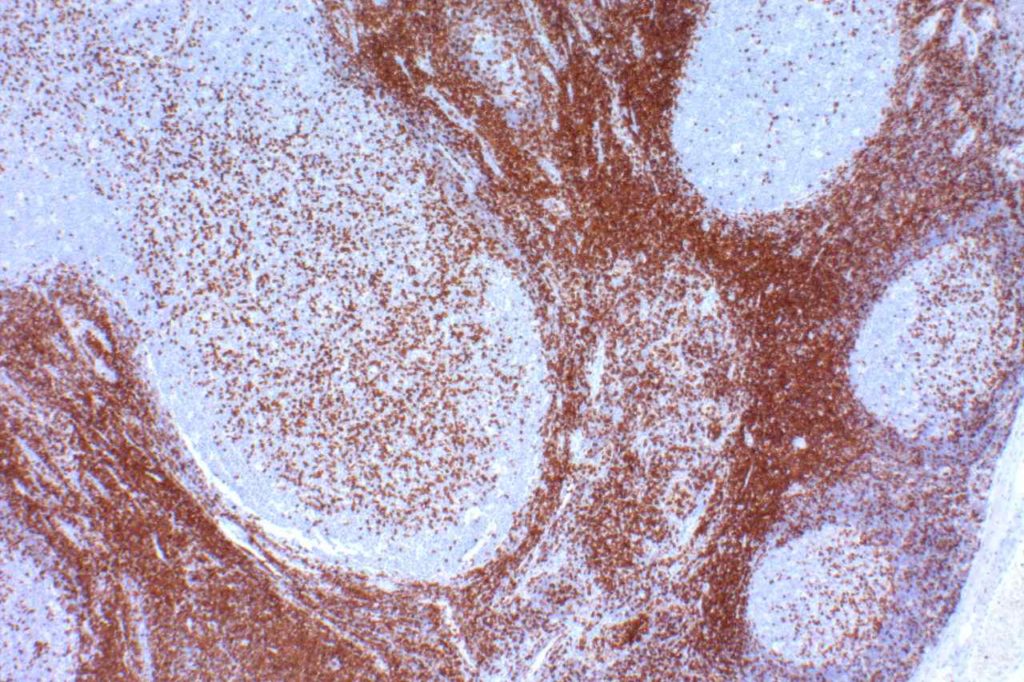
References
Dong HY, Gorczyca W, Liu Z, Tsang P, Wu CD, Cohen P, et al. B-cell lymphomas with coexpression of CD5 and CD10. Am J Clin Pathol. 2003;119: 218–230. doi:10.1309/U98A-DVKU-C26R-2RJA
Gao J, Peterson L, Nelson B, Goolsby C, Chen Y-H. Immunophenotypic variations in mantle cell lymphoma. Am J Clin Pathol. 2009;132: 699–706. doi:10.1309/AJCPV8LN5ENMZOVY
Dalton RR, Admirand JH, Medeiros LJ. Small Lymphocytic Lymphoma. Pathology Case Reviews. 2004;9: 7.
Jaso JM, Yin CC, Wang SA, Miranda RN, Jabcuga CE, Chen L, et al. Clinicopathologic Features of CD5-Positive Nodal Marginal Zone Lymphoma. Am J Clin Pathol. 2013;140: 693–700. doi:10.1309/AJCPEMVXES72DUIF
Went P, Zimpfer A, Tzankov A, Dirnhofer S. CD5 expression in de novo diffuse large B-cell lymphomas. Annals of Oncology. 2009;20: 789–790. doi:10.1093/annonc/mdn793
Cook JR. Nodal and leukemic small B-cell neoplasms. Mod Pathol. 2013;26 Suppl 1: S15–28. doi:10.1038/modpathol.2012.180
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 27.