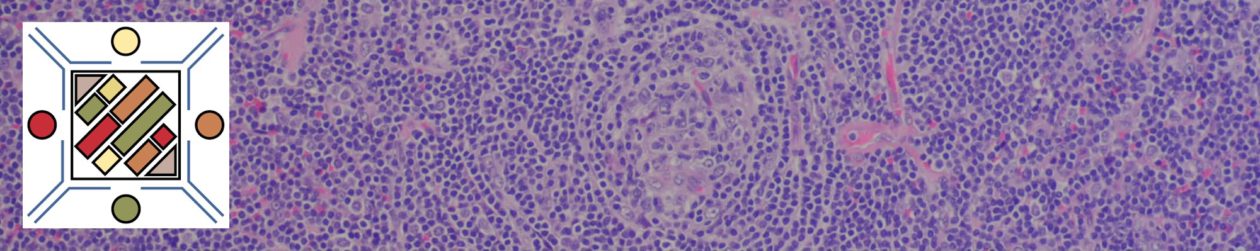
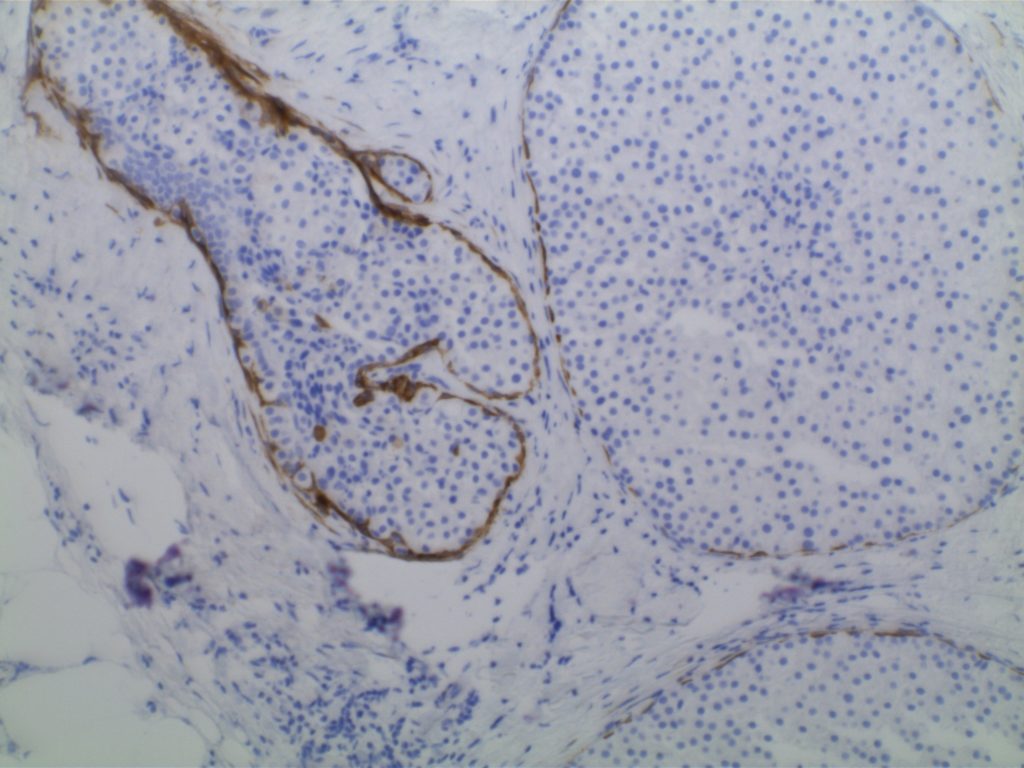
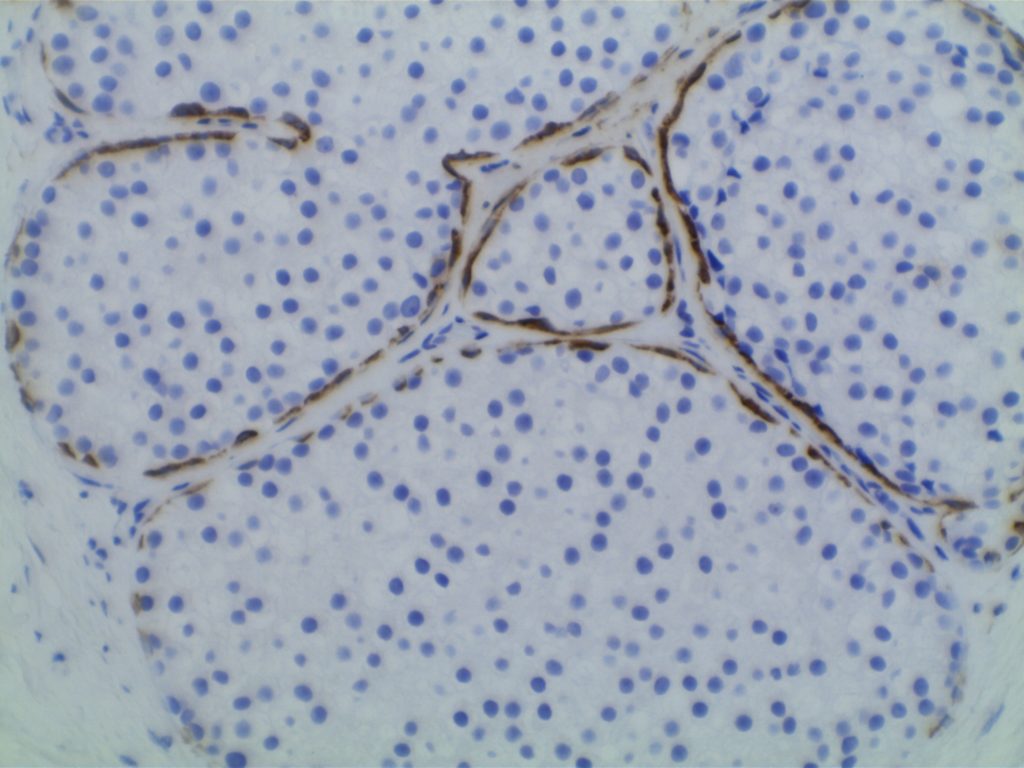
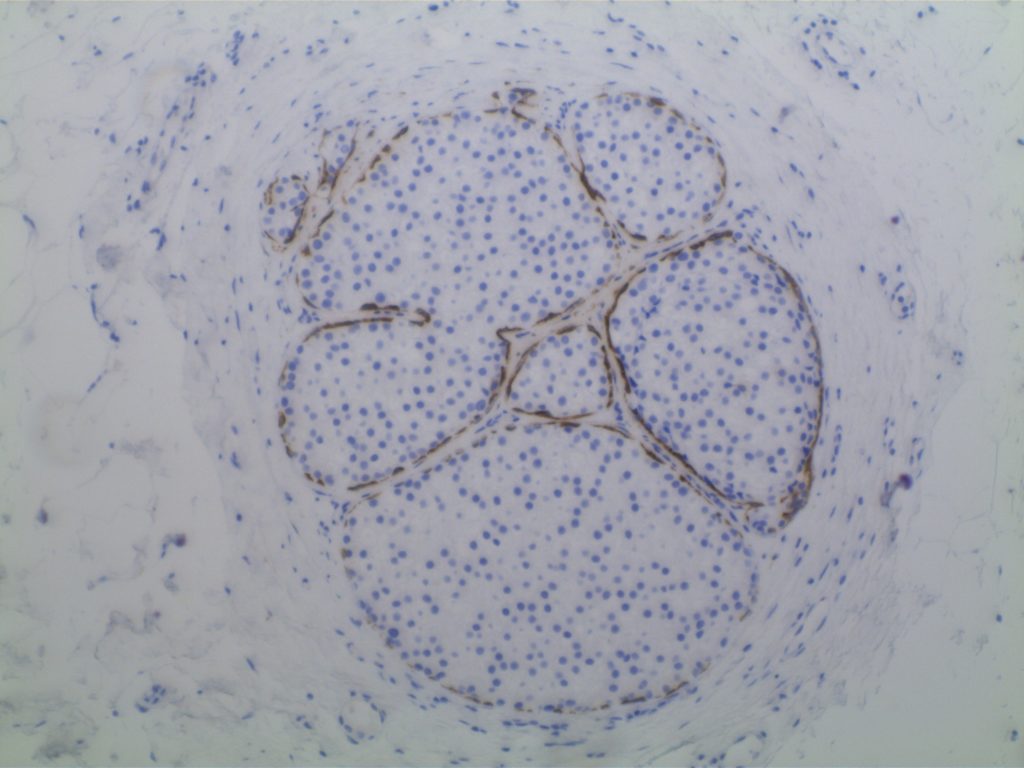
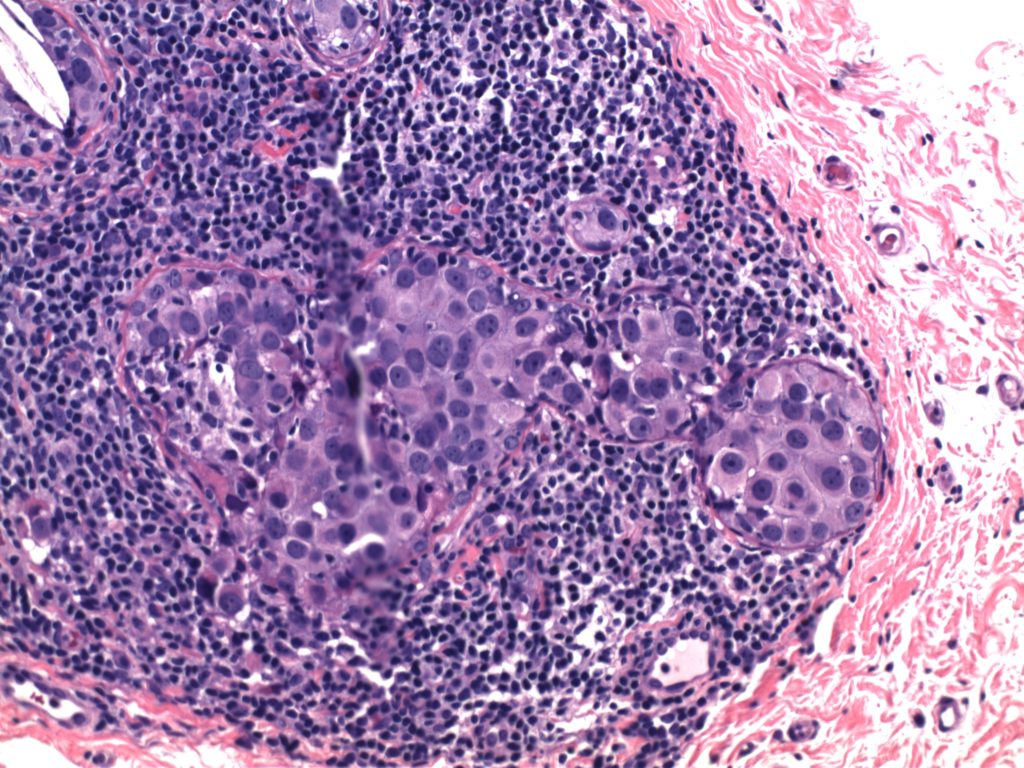
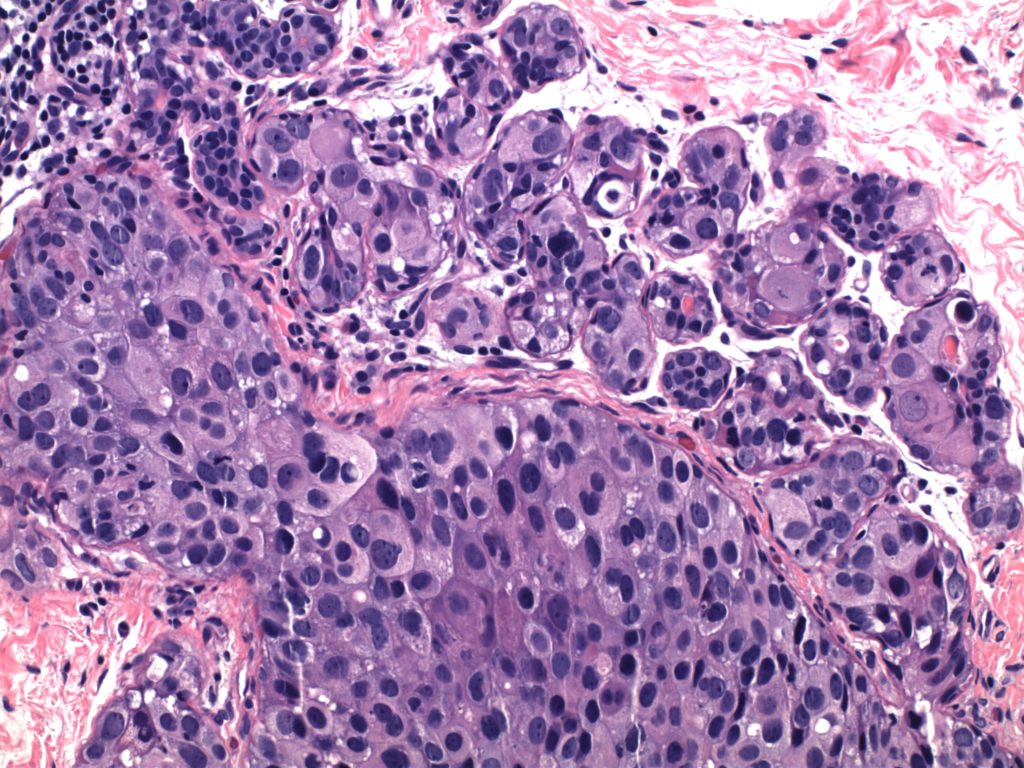
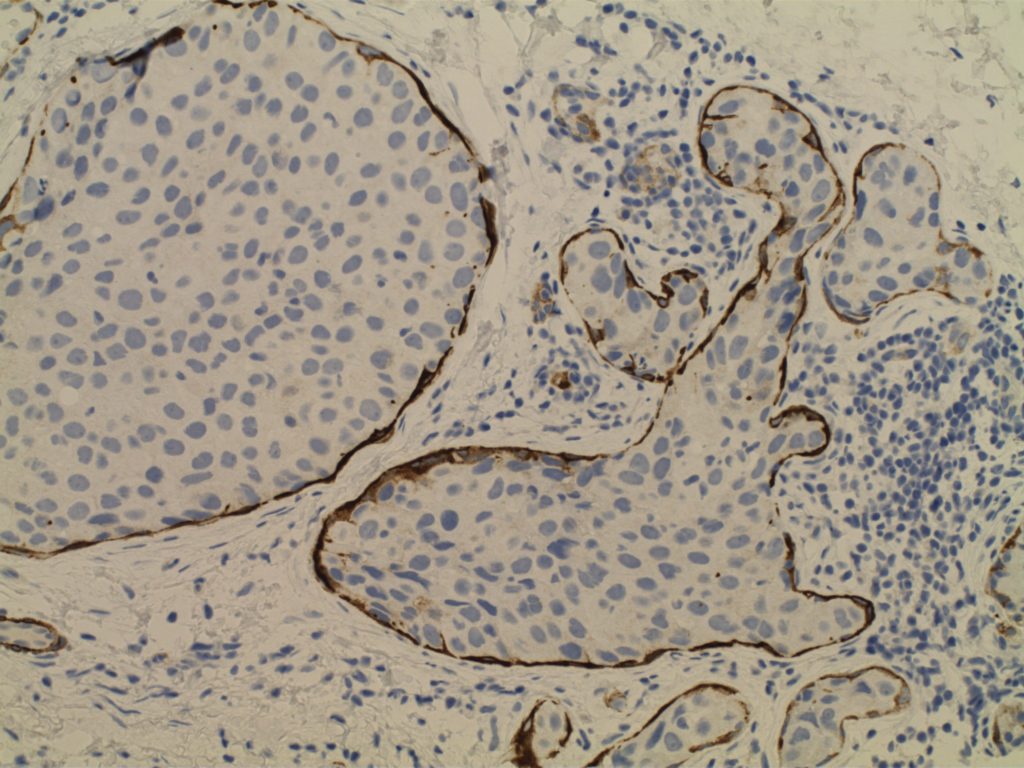
Ductal Carcinoma In Situ (DCIS)
- Neoplastic proliferation resembling small ducts with expanded duct and acinar structures
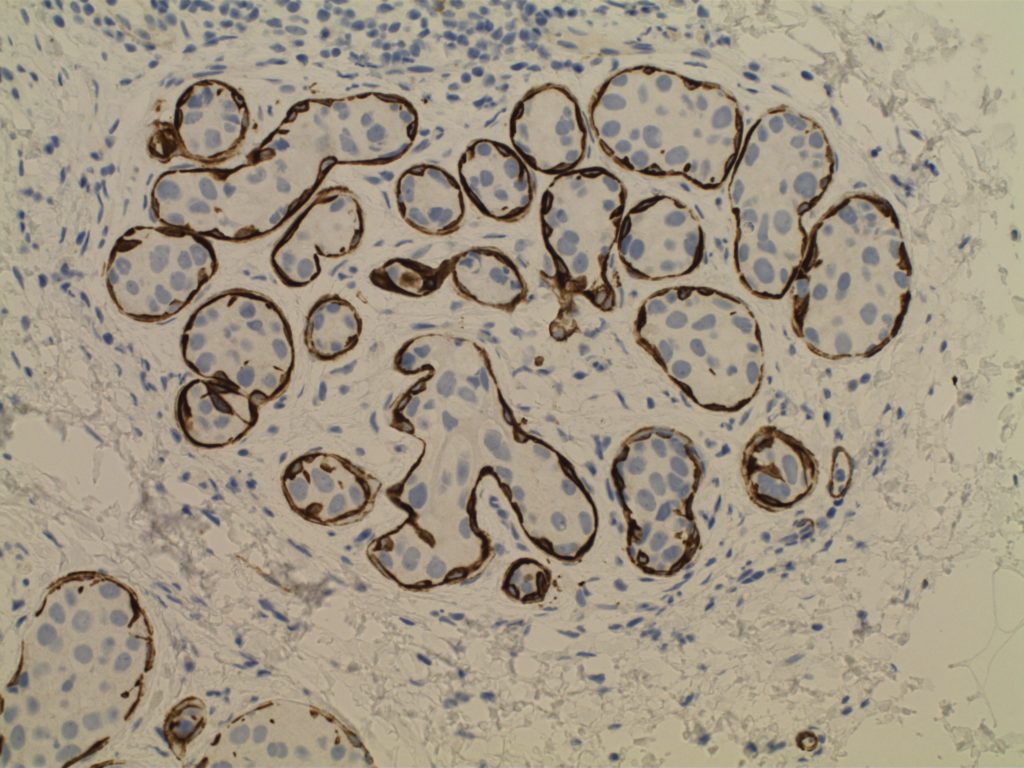
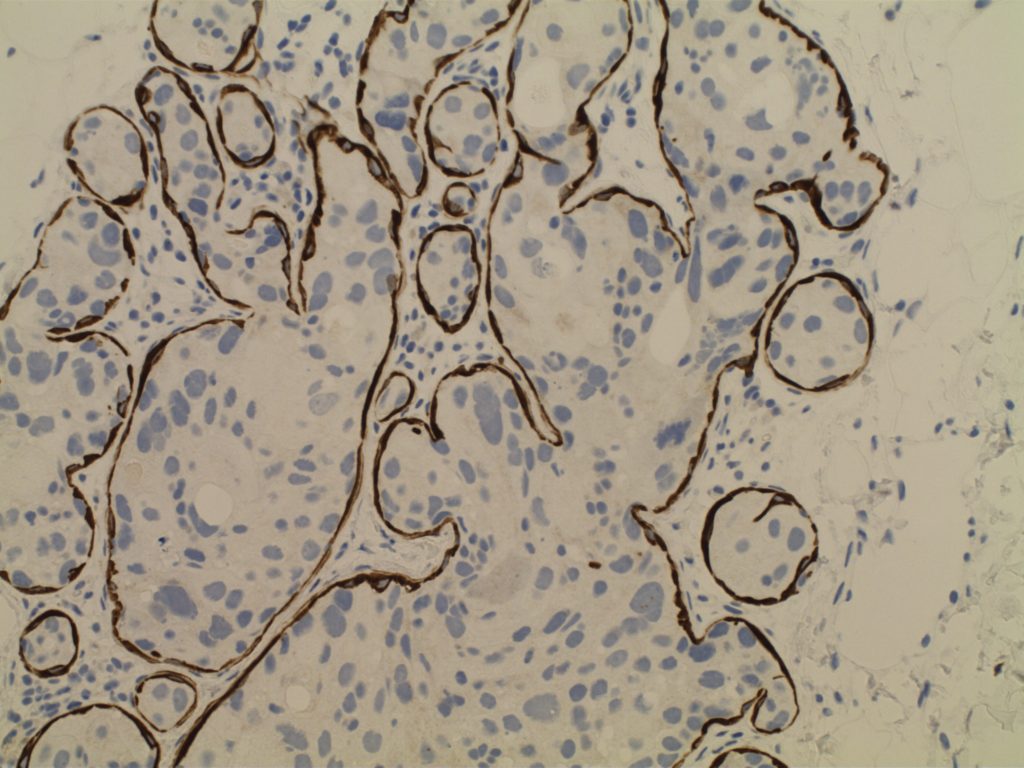
- Myoepithelial cell layer is intact surrounding the neoplastic proliferation (non-invasive tumor)
- Typically express E-Cadherin
- Bilateral in 10-20% of cases
- Detected by mammography (not clinically evident)
- Represents 15-30% of neoplasms identified in screening populations
- Typically identified by abnormal calcification, sometimes as abnormal densities
Comedo DCIS
- High grade pleomorphic nuclei
- Central necrosis
Non-comedo DCIS
- Lacks either high grade nuclei or central necrosis
- Subtypes/patterns
- Solid DCIS
- Micropapillary DCIS
- Cribiform DCIS
- DCIS grading
- Low-grade DCIS
- 1%/year risk of developing an invasive carcinoma
- Intermediate-grade DCIS
- High-grade DCIS
- Low-grade DCIS
Paget Disease
- Nipple manifestation of disease (1-4% of cases) – looks like eczema on the nipple
- Tumor cells extend into the epidermis of the skin overlying the nipple from underlying DCIS within the ductal system of the breast.
- 50-60% of women will have an underlying palpable mass
- Vast majority will have an invasive carcinoma (often ER neg./Her-2 pos.)
- Women without a palpable mass will usually only have DCIS
Photomicrographs
References
Kumar, Vinay, Abul K. Abbas, and Jon C. Aster. Robbins and Cotran Pathologic Basis of Disease. Ninth edition. Philadelphia, PA: Elsevier/Saunders, 2015.