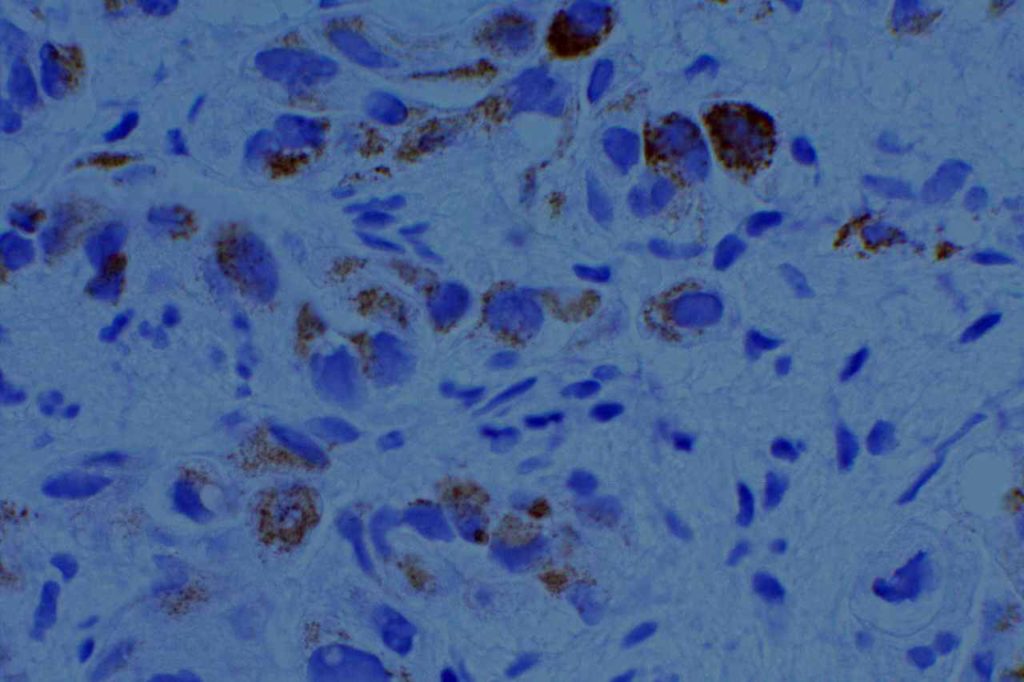
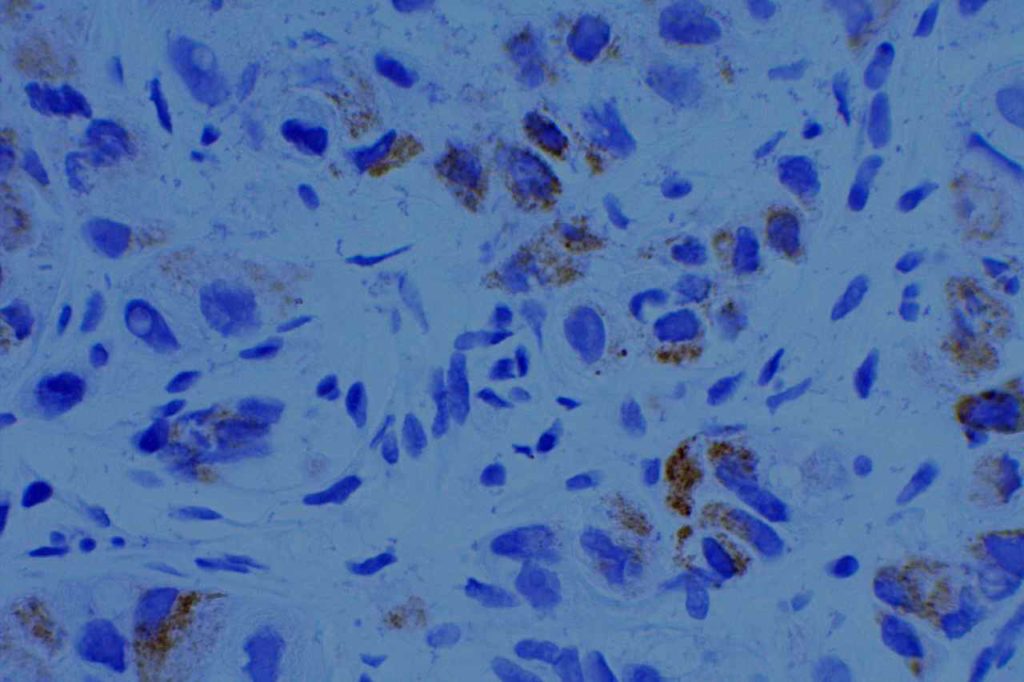
Prostate Adenocarcinoma
IHC Expression Characteristics
|
Negative
|
|
|
Typically negative, rarely positive.
|
|
|
Racemase enzyme expressed by high grade PIN and Prostate adencoarcinoma (NOT a specific marker for prostate tissue). Relatively good specificity (~90%), but may mark benign prostate lesions. Often used in a cocktail with p63 and HMWKs (e.g. PIN4)
|
|
|
>95% sensitivity for prostate adenocarcinoma (highly specific)
|
|
|
PSAP
|
Earlier marker than PSA (now used occasionally as a backup) with less specificity compared to PSA
|
|
Combination stain containing a cocktail of AMACR (red), p63, and high molecular weight cytokeratins (CK5 & CK14)
|
|
|
34BetaE12
|
HMWK that is often used to highlight the basal layer of prostate glands (positivity means it is not invasive adenocarcinoma).
|
|
HMWK which has similar characteristics as 34betaE12
|
|
|
Nuclear marker which highlights the basal layer of benign prostate glands.
|
Prostatic Duct Carcinoma
IHC expression is the same as prostate adenocarcinoma. Morphology is similar to breast DCIS. Sometimes it may be confused with a primary colon adenocarcinoma (CDX-2+) or a primary urothelial carcinoma (PSA=).
References
Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. [edited by] DJ Dabbs. 3rd Edition. Elsevier, 2010.