Lobular Carcinoma In Situ (LCIS)
- Neoplastic proliferation within ducts/lobules (fill and expand lobules)
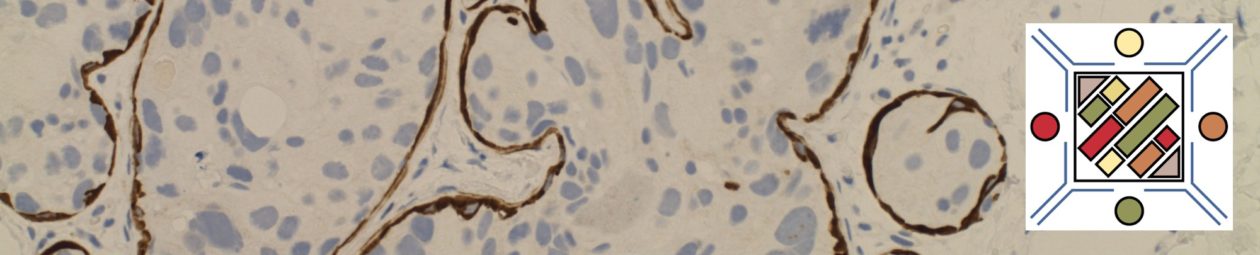
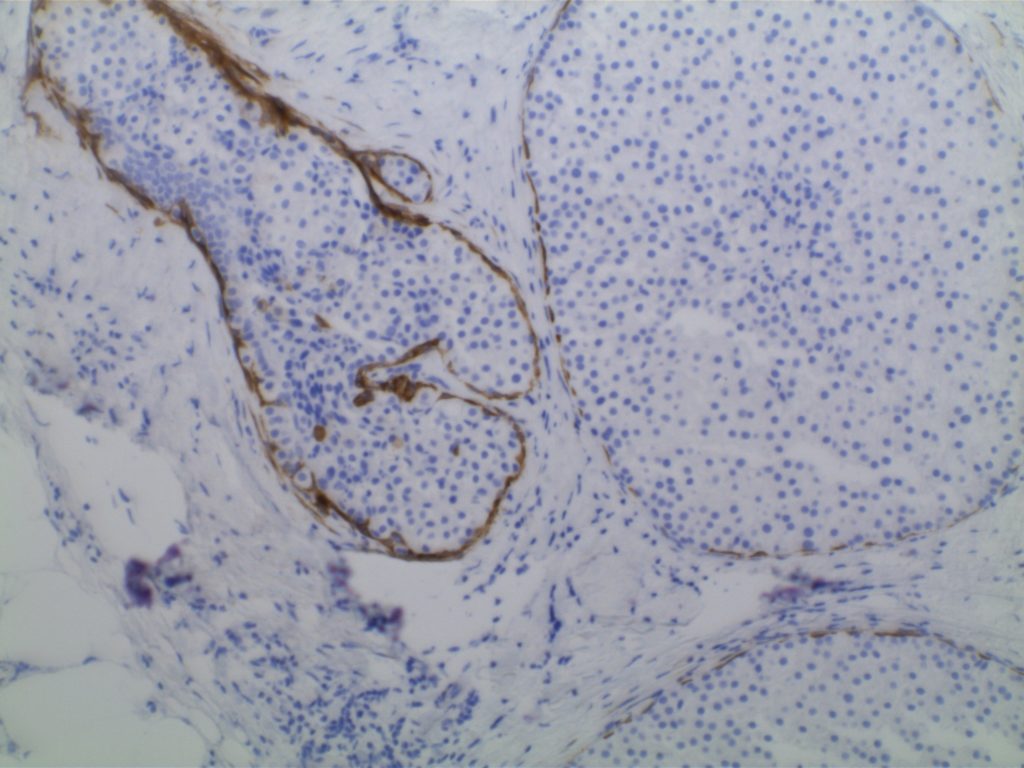
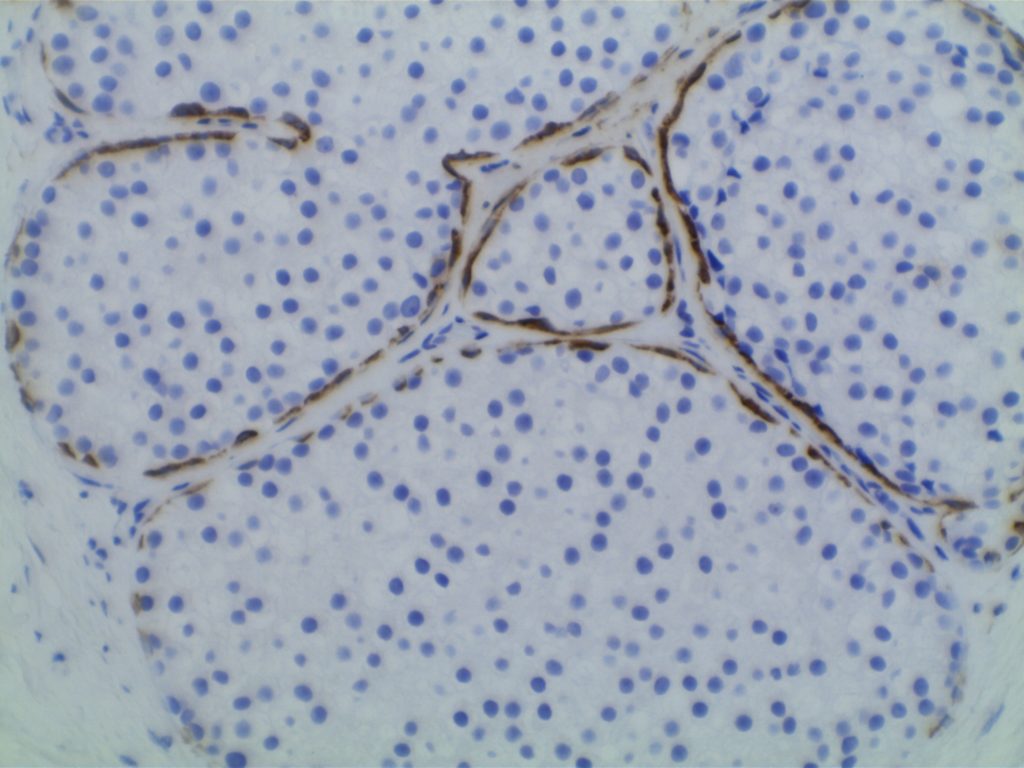
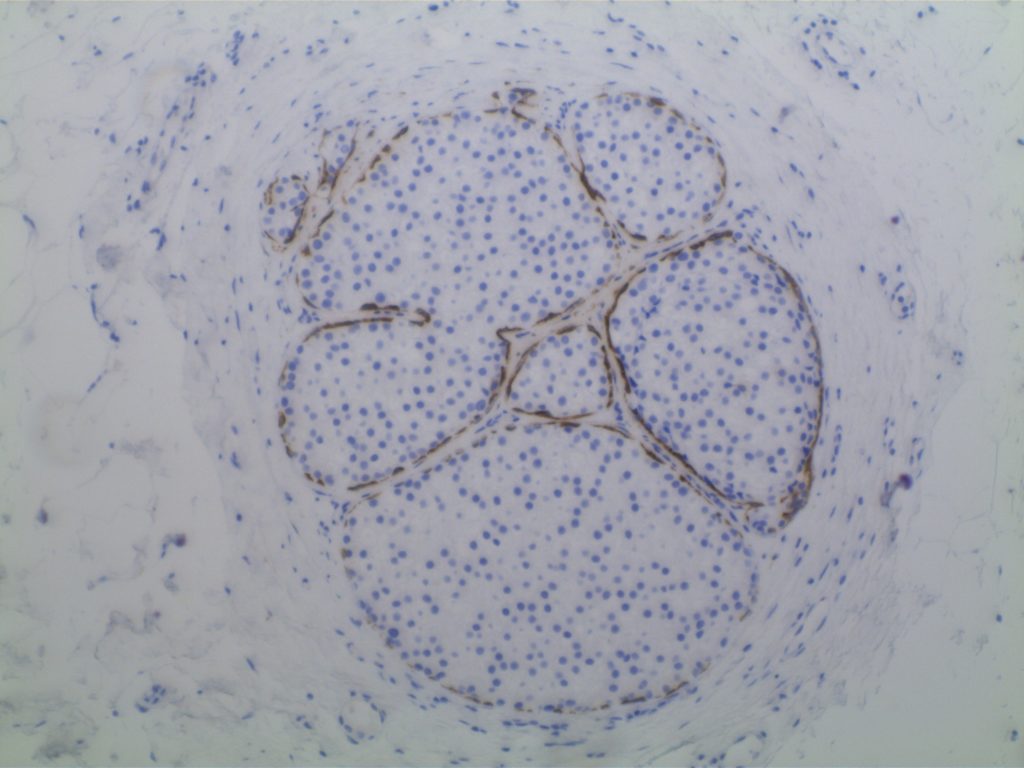
- Associated with loss of expression of E-Cadherin
- Incidental finding (not associated with calcifications or stromal reactions found by mammography)
- LCIS is bilateral 20-40% of cases
- Morphology
- Mucin-positive signet ring cells often present
- E-Cadherin negative cells
- Uniform cells with oval/round nuclei
- Pagetoid spread (cells present between the myoepithelial layer and over lying luminal epithelium
- Typically ER/PR positive
- Her-2 is not overexpressed
- 1%/year risk of developing an invasive tumor (similar to low-grade DCIS) – Lifetime risk is ~25-35% (20-30 year time period)
- Cancer risk is equal in contralateral breast (unlike DCIS)
- Pleomorphic variant of LCIS
- High grade nuclei
- May be ER negative
- Some may overexpress Her-2
- May be separate entity from typical LCIS
Photomicrographs
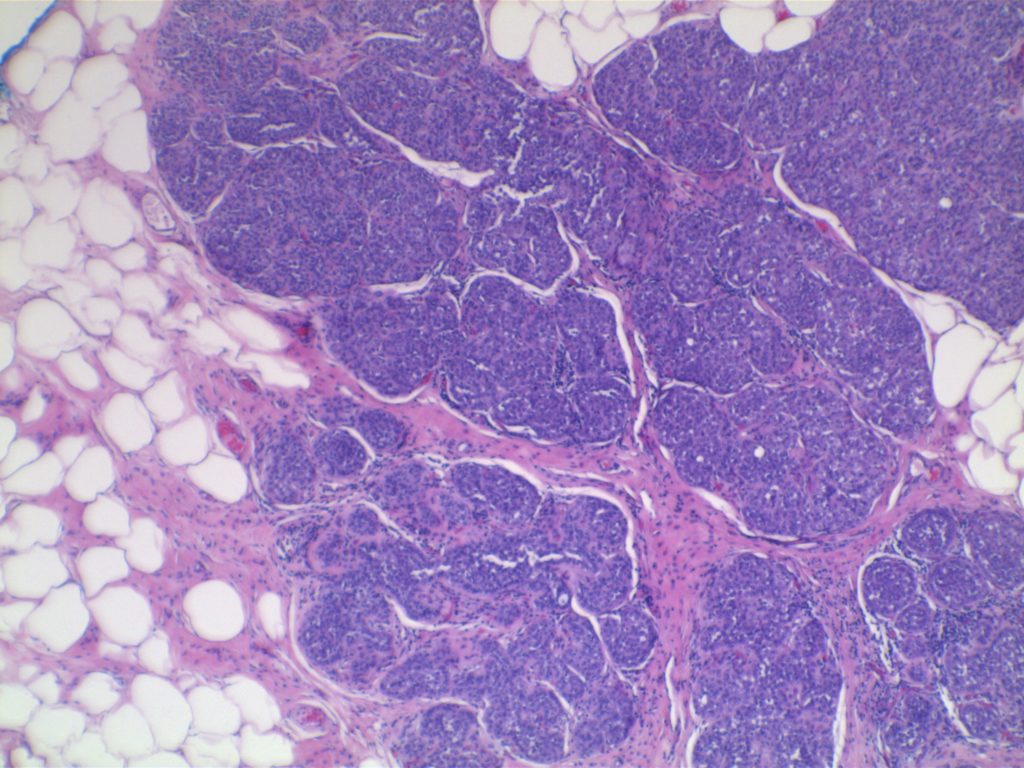
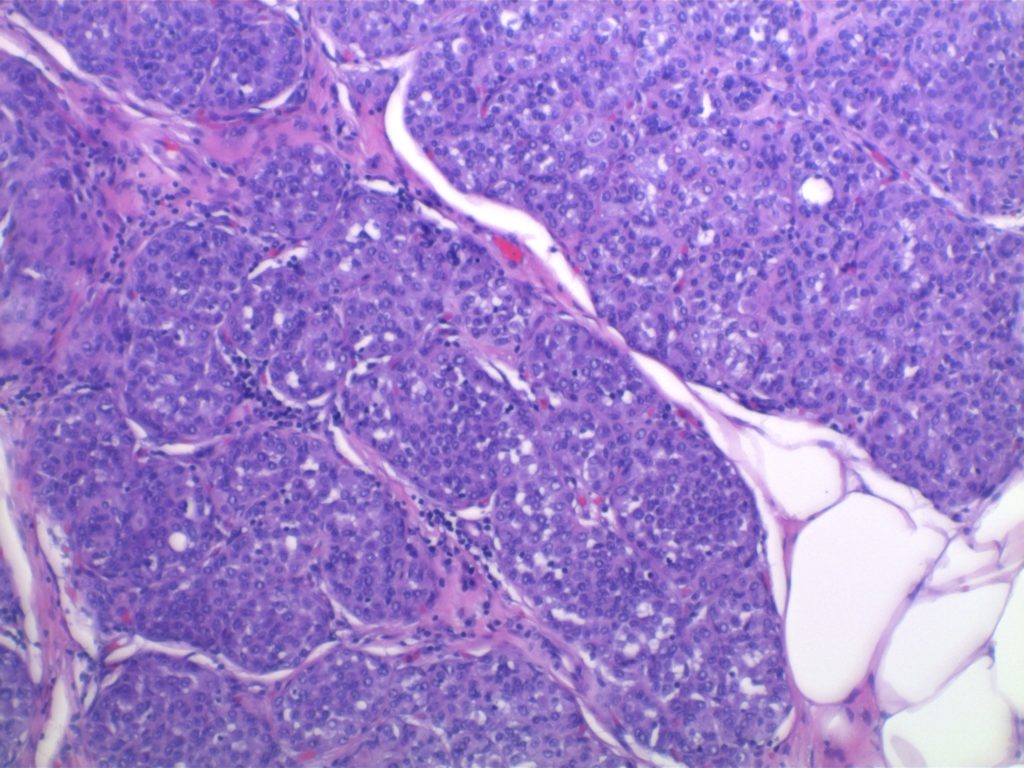
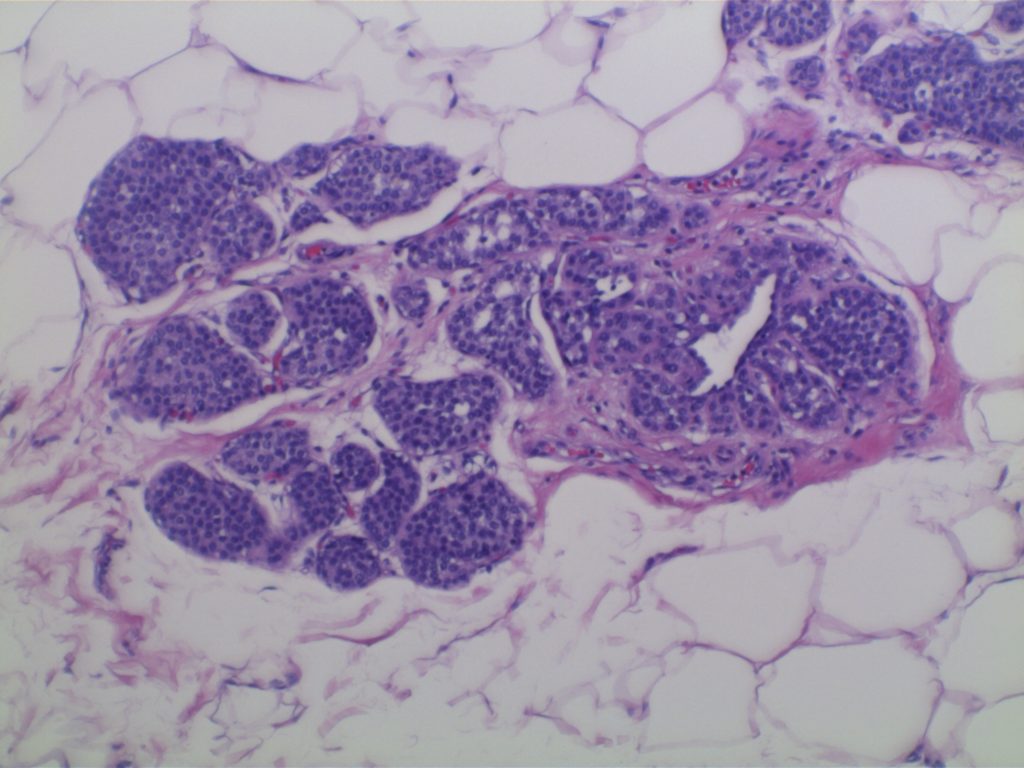
LCIS characterized by filling and expansion of the lobular unit.
References
Kumar, Vinay, Abul K. Abbas, and Jon C. Aster. Robbins and Cotran Pathologic Basis of Disease. Ninth edition. Philadelphia, PA: Elsevier/Saunders, 2015. p. 1045.