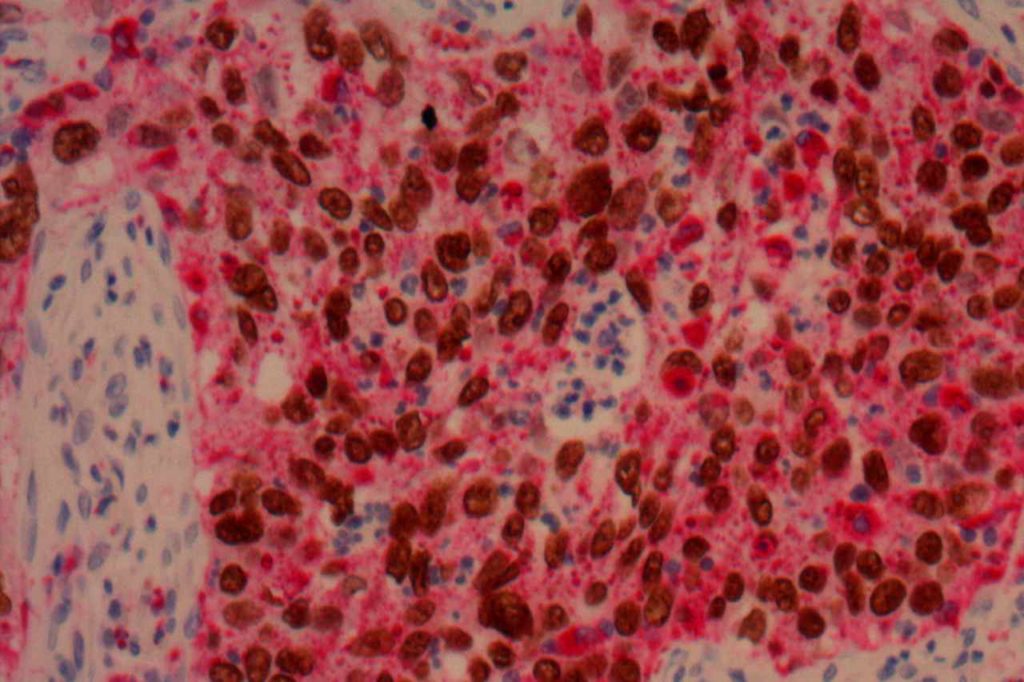
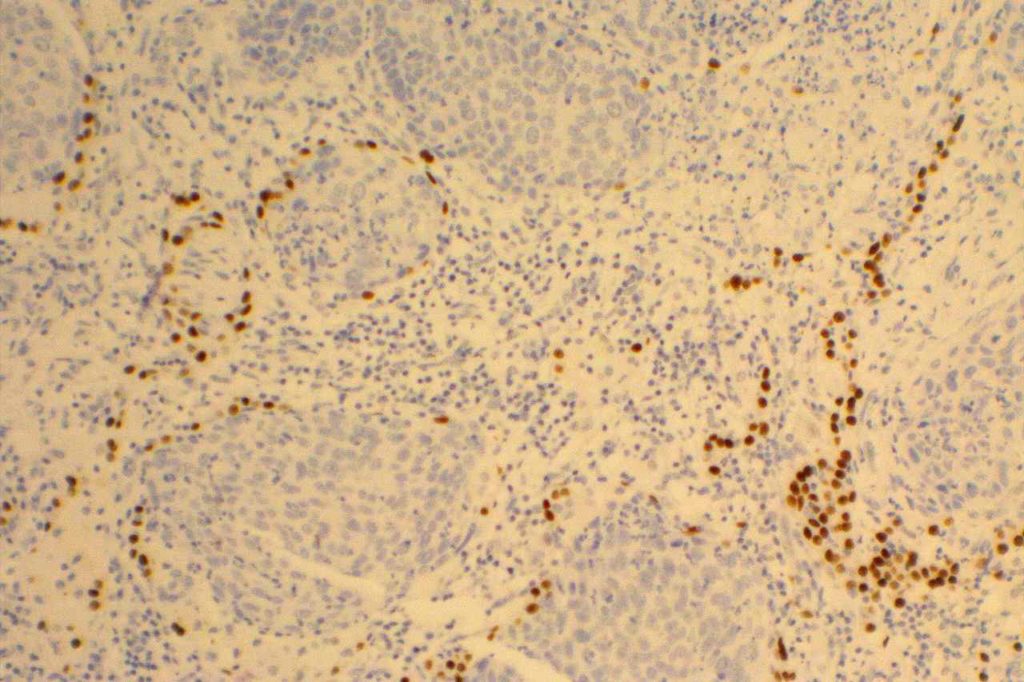
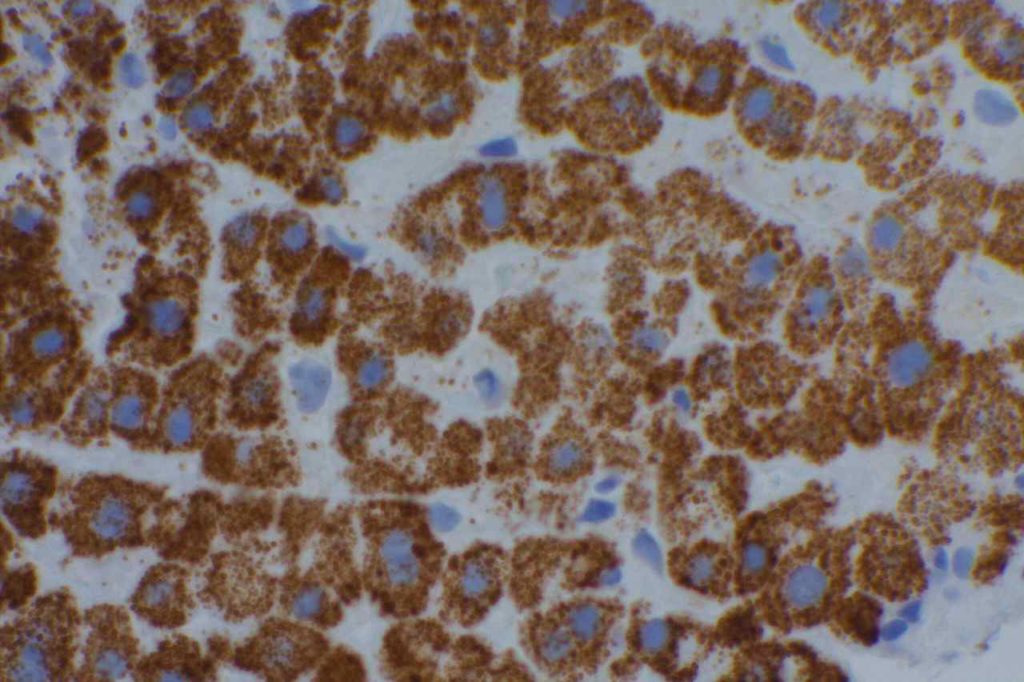
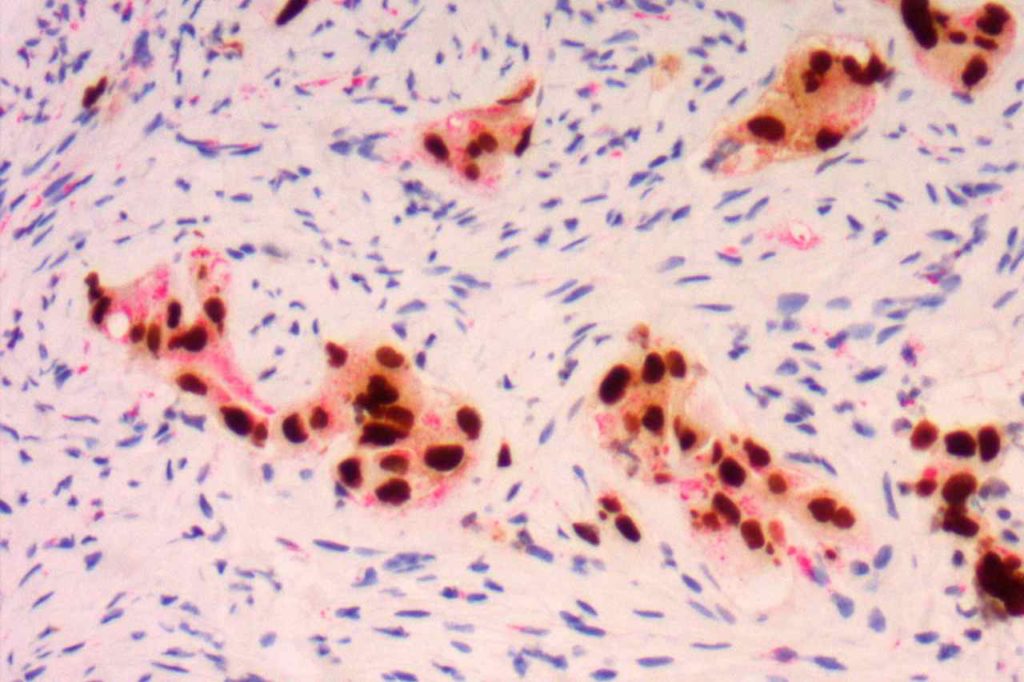
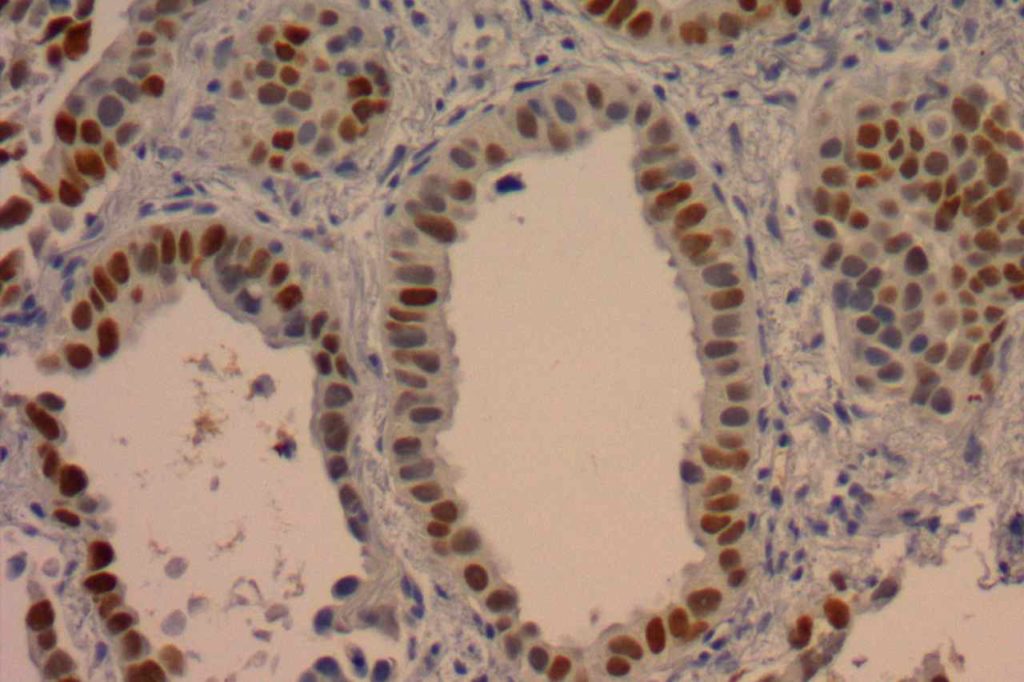
TTF-1 is a nuclear transcription factor that is expressed in thyroid and respiratory epithelium. It is a useful marker for lung adenocarcinomas and thyroid malignancies. In the setting of neuroendocrine carcinomas, TTF-1 expression is not specific as to the site of origin, except that Merkel cell carcinomas of the skin do not usually express TTF-1 (some have reported focal expression). The expression pattern of TTF-1, like other transcription markers, is generally strong and diffuse. Lower levels of positivity should bring caution to the interpretation.
TTF-1 use has become more important to sub-classify lung tumors on small biopsy specimens. It is generally considered the most sensitive and specific individual marker for lung adenocarcinomas, but is often used in combination with Napsin A to maximize sensitivity and specificity for the determination of primary lung adenocarcinomas. Current recommendations are to test non-small cell lung carcinomas (non-squamous cell carcinoma) for ALK, ROS-1 and EGFR mutations, as targeted therapies are available (this list is ever expanding and should be verified with current medical literature).
|
Lung Tumor Subtype
|
Expression (%)
|
|
Squamous Cell Carcinoma
|
0-5%
|
|
Adenocarcinoma
|
80%
|
|
BAC (non-mucinous)
|
90%
|
|
BAC (mucinous)
|
<10%
|
|
Carcinoid Tumor
|
0-35%
|
|
Atypical Carcinoid
|
>85%
|
|
Small Cell Carcinoma
|
>95%
|
|
Large Cell Undifferentiated
|
50-70%
|
Napsin A and TTF-1 expression in poorly differentiated non-small cell carcinomas (Mukjopadhyay, S, et al).
|
Tumor
|
Napsin A
|
TTF-1
|
|
Adenocarcinoma
|
11/19 (58%)
|
16/20 (80%)
|
|
Squamous Cell Carcinoma
|
0/15 (0%)
|
0/15 (0%)
|
|
Large Cell Carcinoma
|
0/4 (0%)
|
2/4 (50%)
|
Common expression patterns in carcinoma (Dennis, et al)
|
Tumor
|
Expression (%)
|
|
Breast
|
0%
|
|
Colon
|
0-10%
|
|
Lung
|
>75%
|
|
Ovary
|
0%
|
|
Pancreas
|
<5%
|
|
Stomach
|
<5%
|
|
Prostate
|
<5%
|
Napsin A and TTF-1 expression in various tumor types (Bishop, JA, et al).
|
Tumor
|
Napsin A
(% positive)
|
TTF-1
(% positive)
|
|
Lung Tumors
|
|
|
|
Adneocarcinoma
|
79/95 (83%)
|
69/95 (73%)
|
|
Well-differentiated
|
42/47 (89%)
|
38/47 (81%)
|
|
Moderately-differentiated
|
27/32 (84%)
|
24/32 (75%)
|
|
Poorly-differentiated
|
11/16 (69%)
|
7/16 (44%)
|
|
Squamous Cell Carcinoma
|
0/46 (0%)
|
0/48 (0%)
|
|
Large Cell Carcinoma
|
3/9 (33%)
|
4/9 (44%)
|
|
Small Cell Carcinoma
|
0/3 (0%)
|
1/3 (33%)
|
|
Atypical Carcinoid Tumor
|
0/1 (0%)
|
1/1 (100%)
|
|
Typical Carcinoid Tumor
|
0/2 (0%)
|
1/2 (50%)
|
|
Nonpulmonary Adenocarcinomas
|
|
|
|
Colon Adenocarcinoma
|
0/5 (0%)
|
0/5 (0%)
|
|
Pancreas Adenocarcinoma
|
0/31 (0%)
|
0/31 (0%)
|
|
Breast Adenocarcinoma
|
0/17 (0%)
|
0/17 (0%)
|
|
Mesothelioma (all types)
|
0/38 (0%)
|
0/38 (0%)
|
|
Renal Cell Carcinomas
|
|
|
|
Clear Cell
|
14/41 (34%)
|
0/41 (0%)
|
|
Papillary
|
34/43 (79%)
|
0/43 (0%)
|
|
Chromophobe
|
1/34 (3%)
|
0/34 (0%)
|
|
Thyroid Lesions
|
|
|
|
Papillary Carcinoma
|
2/38 (5%)
|
37/38 (97%)
|
|
Follicular Carcinoma
|
0/15 (0%)
|
15/15 (100%)
|
|
Follicular Adenoma
|
0/28 (0%)
|
28/28 (100%)
|
Pitfalls
- Don’t forget that thyroid neoplasms can metastasize to the lung (especially follicular carcinomas), which may occur after a significant time period from the original diagnosis. Therefore, in the absence of Napsin A expression, additional staining for thyroglobulin may be helpful to r/o a primary thyroid tumor.
- An additional interesting staining pattern with TTF-1 is in cells of hepatic origin. While they do not express TTF-1, TTF-1 will stain cells of hepatic origin in a granular cytoplasmic pattern similar to HepPar1.
- Recently, Aulakh, KS, et al demonstrated that primary esophageal adenocaricnomas may have similar expression patterns of both Napsin A and TTF-1 compared to primary lung adenocarcinomas, and therefore immunohistochemistry for Napsin A and TTF-1 alone is not effective in separating primary origin in this situation.
- Rare primary breast carcinomas may express TTF-1 (2-3% in large study sets).
- Occasional cases of endometrial adenocarcinoma may express TTF-1 (~17%).
Normal Expression Pattern
- Lung
- Alveolar epithelium
- Non-ciliated respiratory epithelium
- Thyroid
- Follicle cells
- C-cells
- Parathyroid
- Pituitary (anterior)
- Brain (diencephalon)
Photomicrographs
References:
Hadi, AIMM Annual Meeting, “Carcinomas of Unknown Primary”, presentation, 2011.
Bishop, J. A., Sharma, R., & Illei, P. B. (2010). Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Human Pathology, 41(1), 20–25. doi:10.1016/j.humpath.2009.06.014
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236
Inamura, K., Takeuchi, K., Togashi, Y., Hatano, S., Ninomiya, H., Motoi, N., et al. (2009). EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Modern Pathology : an Official Journal of the United States and Canadian Academy of Pathology, Inc, 22(4), 508–515. doi:10.1038/modpathol.2009.2
Aulakh, K. S., Chisholm, C. D., Smith, D. A., & Speights, V. O. (2013). TTF-1 and napsin A do not differentiate metastatic lung adenocarcinomas from primary esophageal adenocarcinomas: proposal of a novel staining panel. Archives of Pathology & Laboratory Medicine, 137(8), 1094–1098. doi:10.5858/arpa.2012-0305-OA
Mukhopadhyay, S., & Katzenstein, A.-L. A. (2011). Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: Utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. The American Journal of Surgical Pathology, 35(1), 15–25. doi:10.1097/PAS.0b013e3182036d05